(1E)-1-Propen-1-ylboronic acid
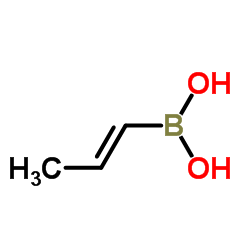
(1E)-1-Propen-1-ylboronic acid structure
|
Common Name | (1E)-1-Propen-1-ylboronic acid | ||
|---|---|---|---|---|
| CAS Number | 7547-97-9 | Molecular Weight | 85.897 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 175.6±23.0 °C at 760 mmHg | |
| Molecular Formula | C3H7BO2 | Melting Point | 123-127ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 60.0±22.6 °C | |
| Name | (E)-Prop-1-en-1-ylboronic acid |
|---|---|
| Synonym | More Synonyms |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 175.6±23.0 °C at 760 mmHg |
| Melting Point | 123-127ºC(lit.) |
| Molecular Formula | C3H7BO2 |
| Molecular Weight | 85.897 |
| Flash Point | 60.0±22.6 °C |
| Exact Mass | 86.053909 |
| PSA | 40.46000 |
| LogP | 0.96 |
| Vapour Pressure | 0.4±0.7 mmHg at 25°C |
| Index of Refraction | 1.422 |
| Storage condition | -20 °C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
Discovery of potent, selective, and orally bioavailable alkynylphenoxyacetic acid CRTH2 (DP2) receptor antagonists for the treatment of allergic inflammatory diseases.
J. Med. Chem. 20th ed., 54 , 7299-7317, (2011) New phenoxyacetic acid antagonists of CRTH2 are described. Following the discovery of a hit compound by a focused screening, high protein binding was identified as its main weakness. Optimization aime... |
|
|
Selective palladium-catalyzed C-F activation/carbon-carbon bond formation of polyfluoroaryl oxazolines.
J. Org. Chem. 4th ed., 77 , 1798-1804, (2012) A selective palladium-catalyzed Suzuki-Miyaura coupling reaction of polyfluorophenyl oxazolines through ortho C-F activation is described. It was found that reactions with DPPF as the ligand occurred ... |
|
|
Highly selective nickel-catalyzed three-component coupling of alkynes with enones and alkenyl boronic acids: a novel route to substituted 1,3-dienes.
Org. Lett. 12 , 3610-3613, (2010) A highly regio- and stereoselective nickel-catalyzed three-component coupling of alkynes with enones and alkenyl boronic acids to afford highly substituted 1,3-dienes is described. The reaction can al... |
| (1E)-1-Propen-1-ylboronic acid |
| [(E)-prop-1-enyl]boronic acid |
| Boronic acid, B-[(1E)-1-propen-1-yl]- |