Quassin
Modify Date: 2024-01-02 17:05:38
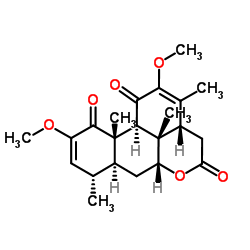
Quassin structure
|
Common Name | Quassin | ||
|---|---|---|---|---|
| CAS Number | 76-78-8 | Molecular Weight | 388.454 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 586.3±50.0 °C at 760 mmHg | |
| Molecular Formula | C22H28O6 | Melting Point | 200 - 222ºC | |
| MSDS | N/A | Flash Point | 255.4±30.2 °C | |
Use of QuassinQuassin (Nigakilactone D) is a bioactive triterpenoid from stem bark extract of Quassia amara. Quassin inhibits P. falciparum with an IC50 of 0.15 μM. Quassin possesses reversible antifertility, anti-estrogenic and anti-plasmodial activity[1][2]. |
| Name | quassin |
|---|---|
| Synonym | More Synonyms |
| Description | Quassin (Nigakilactone D) is a bioactive triterpenoid from stem bark extract of Quassia amara. Quassin inhibits P. falciparum with an IC50 of 0.15 μM. Quassin possesses reversible antifertility, anti-estrogenic and anti-plasmodial activity[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.15 μM (P. falciparum)[2] |
| In Vitro | Quassin (Compound 1; 5-25 ng/mL) inhibits both the basal and luteinizing hormone-stimulated testosterone secretion of rat Leydig cells in a dose-dependent fashion[3]. |
| In Vivo | Quassin (0.1-2.0 mg/kg; oral administration; daily; for 60 days; females albino rats) treatment shows a significant decrease in the weight of the ovary and uterus. And also shows a significant decrease in serum estrogen levels in quassin treated rats. The Quassin treated rats has a significantly decreased mean litter number and weight. Quassin does not adversely affect the weight of the kidney, heart, liver and the body of the rats[1]. Animal Model: 35 females albino rats (150-170 g)[1] Dosage: 0.1 mg/kg, 1.0 mg/kg and 2.0 mg/kg Administration: Oral administration; daily; for 60 days Result: There was a significant decrease in the weight of the ovary and uterus in all the groups relative to the control. There was also a significant decrease in serum estrogen levels in quassin treated rats. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 586.3±50.0 °C at 760 mmHg |
| Melting Point | 200 - 222ºC |
| Molecular Formula | C22H28O6 |
| Molecular Weight | 388.454 |
| Flash Point | 255.4±30.2 °C |
| Exact Mass | 388.188599 |
| PSA | 78.90000 |
| LogP | 2.22 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.553 |
| Storage condition | 2-8℃ |
| Hazard Codes | Xi |
|---|
| Precursor 0 | |
|---|---|
| DownStream 2 | |
| Einecs 200-985-9 |
| Picrasa-2,12-diene-1,11,16-trione, 2,12-dimethoxy- |
| 2,12-Dimethoxypicrasa-2,12-diene-1,11,16-trione |
| Quassin,mixture of isomers w/Neoquassin and Isoquassin |
| 11,16-trione,2,12-dimethoxy-picrasa-12-diene-1 |
| Picrasa-2,12-diene-1,11,16-trione,2,12-dimethoxy |
| Nigakilactone D |
| Quassin |
| (3aR,6aR,7aS,8S,11aS,11bS,11cS)-2,10-Dimethoxy-3,8,11a,11c-tetramethyl-3a,6a,7,7a,8,11a,11b,11c-octahydrodibenzo[de,g]chromene-1,5,11(4H)-trione |
| 3ab,6ab,7,7aa,8,11a,11ba,11c-Octahydro-2,10-dimethoxy-3,8a,11ab,11cb-tetramethylphenanthro[10,1-bc]pyran-1,5,11(4H)-trione |
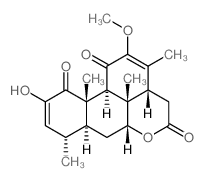 CAS#:26121-57-3
CAS#:26121-57-3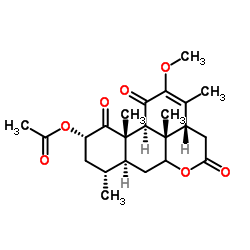 CAS#:30315-04-9
CAS#:30315-04-9
