Azamulin
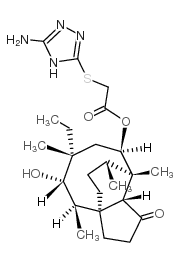
Azamulin structure
|
Common Name | Azamulin | ||
|---|---|---|---|---|
| CAS Number | 76530-44-4 | Molecular Weight | 478.64800 | |
| Density | 1.27g/cm3 | Boiling Point | 659.4ºC at 760 mmHg | |
| Molecular Formula | C24H38N4O4S | Melting Point | 128-130ºC | |
| MSDS | Chinese USA | Flash Point | 352.6ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of AzamulinAzamulin is an irreversible, highly selective inhibitior of human CYP3Aa. Azamulin has CYP3A inhibition activity with IC50 values range from 0.03-0.24 μM. Azamulin can be used for the research of metabolism and antiinfection[1]. |
| Name | Azamulin |
|---|---|
| Synonym | More Synonyms |
| Description | Azamulin is an irreversible, highly selective inhibitior of human CYP3Aa. Azamulin has CYP3A inhibition activity with IC50 values range from 0.03-0.24 μM. Azamulin can be used for the research of metabolism and antiinfection[1]. |
|---|---|
| Related Catalog | |
| Target |
CYP3A:0.03-0.24 μM (IC50) |
| In Vitro | Azamulin 具有 CYP3A 抑制活性,IC50 值范围为 0.03-0.24 μM[1]。 Azamulin 对 CYP3A 的抑制呈 S 型曲线,并且对底物 7-benzyloxy-4-trifluoromethylcoumarin, testosterone 和 midazolam 表现良好[1]。 Azamulin (4.8 μM; 10 min) 在 NADPH 存在的情况下抑制约 95% 的睾酮 6β-羟化酶活性[1]。 Azamulin 在乙腈中储存长达 12 天时表现出良好的化学稳定性[1]。 |
| References |
| Density | 1.27g/cm3 |
|---|---|
| Boiling Point | 659.4ºC at 760 mmHg |
| Melting Point | 128-130ºC |
| Molecular Formula | C24H38N4O4S |
| Molecular Weight | 478.64800 |
| Flash Point | 352.6ºC |
| Exact Mass | 478.26100 |
| PSA | 156.49000 |
| LogP | 4.19070 |
| Index of Refraction | 1.59 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| RIDADR | NONH for all modes of transport |
|
Chemical inhibitors of CYP450 enzymes in liver microsomes: combining selectivity and unbound fractions to guide selection of appropriate concentration in phenotyping assays.
Xenobiotica 45(2) , 95-106, (2014) 1. Chemical inhibition is the widely used method in reaction phenotyping assays for estimation of specific enzyme contribution to a given metabolic pathway. The results from phenotyping assays depend ... |
| (3aS,4R,5S,6R,8R,9R,9aR,10R)-6-Ethyldecahydro-5-hydroxy-4,6,9,10-tetramethyl-1-oxo-3a,9-propanocyclopentacycloocten-8-yl ((5-amino-1,2,4-triazol-3-yl)thio)acetat |
| ((5-Amino-s-triazol-3-yl)thio)acetic acid,8-ester with (3aS,4R,5S,6R,8R,9aR,10R)-6-ethyloctahydro-5,8-dihydroxy-4,6,9,10-tetramethyl-3a,9-propano-3aH-cyclopentacycloocten-1(4H)-one |
| UNII-875AQ866X1 |