Ethyl 1H-indole-3-carboxylate
Modify Date: 2024-01-05 10:26:43

Ethyl 1H-indole-3-carboxylate structure
|
Common Name | Ethyl 1H-indole-3-carboxylate | ||
|---|---|---|---|---|
| CAS Number | 776-41-0 | Molecular Weight | 189.210 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 342.4±15.0 °C at 760 mmHg | |
| Molecular Formula | C11H11NO2 | Melting Point | 120-124ºC | |
| MSDS | Chinese USA | Flash Point | 160.9±20.4 °C | |
| Name | Ethyl Indole-3-Carboxylate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 342.4±15.0 °C at 760 mmHg |
| Melting Point | 120-124ºC |
| Molecular Formula | C11H11NO2 |
| Molecular Weight | 189.210 |
| Flash Point | 160.9±20.4 °C |
| Exact Mass | 189.078979 |
| PSA | 42.09000 |
| LogP | 3.07 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.621 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| Precursor 9 | |
|---|---|
| DownStream 8 | |
|
Synthesis of the aziridinomitosene skeleton by intramolecular Michael addition of alpha-lithioaziridines: an aromatic route featuring deuterium as a removable blocking group.
J. Org. Chem. 69 , 1794-1799, (2004) A convergent synthetic route to the 1,2-aziridinopyrrolo(1,2-a)indole 34 has been developed. Key features of this route include the deuterium kinetic isotope effect to block undesired indole lithiatio... |
| 1H-Indole-3-carboxylic acid, ethyl ester |
| MFCD00228454 |
| Ethyl 1H-indole-3-carboxylate |
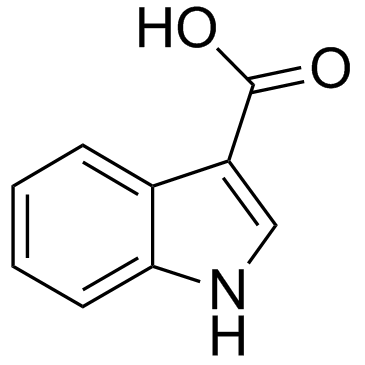 CAS#:771-50-6
CAS#:771-50-6 CAS#:75-03-6
CAS#:75-03-6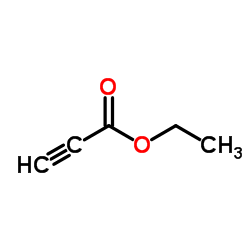 CAS#:623-47-2
CAS#:623-47-2 CAS#:64-17-5
CAS#:64-17-5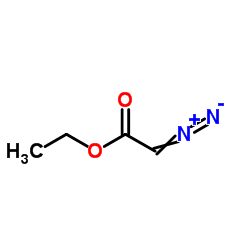 CAS#:623-73-4
CAS#:623-73-4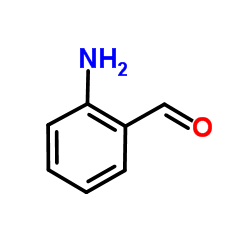 CAS#:529-23-7
CAS#:529-23-7 CAS#:586-96-9
CAS#:586-96-9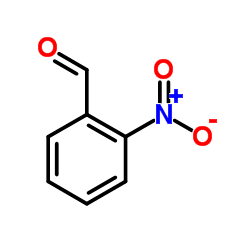 CAS#:552-89-6
CAS#:552-89-6 CAS#:615-43-0
CAS#:615-43-0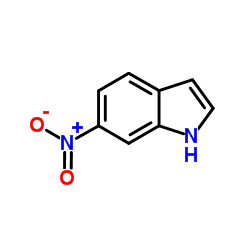 CAS#:4769-96-4
CAS#:4769-96-4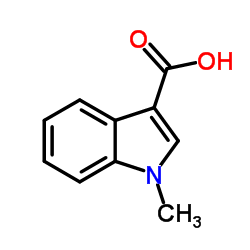 CAS#:32387-21-6
CAS#:32387-21-6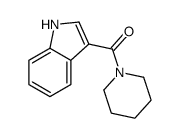 CAS#:27393-79-9
CAS#:27393-79-9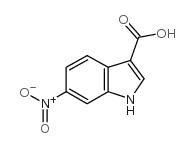 CAS#:10242-03-2
CAS#:10242-03-2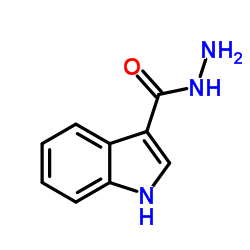 CAS#:15317-58-5
CAS#:15317-58-5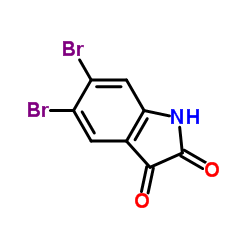 CAS#:17826-05-0
CAS#:17826-05-0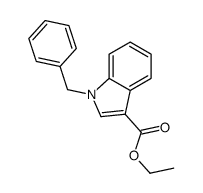 CAS#:56559-61-6
CAS#:56559-61-6 CAS#:56559-62-7
CAS#:56559-62-7
