Iodic acid
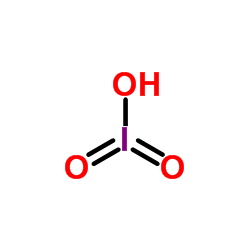
Iodic acid structure
|
Common Name | Iodic acid | ||
|---|---|---|---|---|
| CAS Number | 7782-68-5 | Molecular Weight | 175.911 | |
| Density | 4.62 | Boiling Point | N/A | |
| Molecular Formula | HIO3 | Melting Point | 110ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |


GHS03, GHS05 |
Signal Word | Danger | |
| Name | iodic acid |
|---|---|
| Synonym | More Synonyms |
| Density | 4.62 |
|---|---|
| Melting Point | 110ºC |
| Molecular Formula | HIO3 |
| Molecular Weight | 175.911 |
| Exact Mass | 175.897034 |
| PSA | 54.37000 |
| LogP | 0.47130 |
| Index of Refraction | 1.95 |
| Stability | Stable. Incompatible with strong reducing agents, alcohols, organic materials. Light-sensitive. |
| Water Solubility | 269 g/100 mL (20 ºC) |
Synonym:Hydrogen Iodat Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: 34 8 Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Causes burns. Contact with combustible material may cause fire.Corrosive. Potential Health Effects Eye: Causes eye burns. May cause permanent corneal opacification. May cause chemical conjunctivitis and corneal damage. Skin: Causes skin burns. May cause skin rash (in milder cases), and cold and clammy skin with cyanosis or pale color. Ingestion: May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract burns. May cause perforation of the digestive tract. May cause systemic effects. May cause nausea, vomiting, and diarrhea, possibly with blood. Inhalation: Causes chemical burns to the respiratory tract. May cause systemic effects. May cause acute pulmonary edema, asphyxia, chemical pneumonitis, and upper airway obstruction caused by edema. Chronic: Effects may be delayed. Section 4 - FIRST AID MEASURES Eyes: Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed. Extensive irrigation with water is required (at least 30 minutes). Skin: Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes. Ingestion: Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Get medical aid immediately. Inhalation: Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask. Notes to Physician: Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Strong oxidizer. Contact with other material may cause fire. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Substance is noncombustible. Use water with caution and in flooding amounts. Oxidizer. Greatly increases the burning rate of combustible materials. Extinguishing Media: Substance is noncombustible; use agent most appropriate to extinguish surrounding fire. Contact professional fire-fighters immediately. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Wear a self contained breathing apparatus and appropriate personal protection. (See Exposure Controls, Personal Protection section). Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation. Section 7 - HANDLING and STORAGE Handling: Remove contaminated clothing and wash before reuse. Minimize dust generation and accumulation. Do not get in eyes, on skin, or on clothing. Keep container tightly closed. Avoid contact with clothing and other combustible materials. Avoid ingestion and inhalation. Use with adequate ventilation. Discard contaminated shoes. Storage: Keep away from heat, sparks, and flame. Do not store near combustible materials. Do not store in direct sunlight. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Corrosives area. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 7782-68-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate gloves to prevent skin exposure. Clothing: Wear a chemical apron. Wear appropriate clothing to prevent skin exposure. Respirators: Wear a NIOSH/MSHA or European Standard EN 149 approved full-facepiece airline respirator in the positive pressure mode with emergency escape provisions. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Solid Color: white to pale yellow Odor: none reported pH: Acidic in solution. Vapor Pressure: Negligible. Viscosity: Not available. Boiling Point: Not available. Freezing/Melting Point: 230 deg F Autoignition Temperature: Not applicable. Flash Point: Not applicable. Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: 230 deg F Solubility in water: 2.8 kg/L H2O @ 0C Specific Gravity/Density: 4.629 (water=1) Molecular Formula: HIO3 Molecular Weight: 175.9097 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable under normal temperatures and pressures. Conditions to Avoid: Incompatible materials, light, ignition sources, dust generation, combustible materials, reducing agents. Incompatibilities with Other Materials: Reducing agents. Hazardous Decomposition Products: Irritating and toxic fumes and gases, iodine. Hazardous Polymerization: Has not been reported. Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 7782-68-5 unlisted. LD50/LC50: Not available. Carcinogenicity: Iodic Acid - Not listed by ACGIH, IARC, or NTP. Section 12 - ECOLOGICAL INFORMATION Section 13 - DISPOSAL CONSIDERATIONS Products which are considered hazardous for supply are classified as Special Waste and the disposal of such chemicals is covered by regulations which may vary according to location. Contact a specialist disposal company or the local waste regulator for advice. Empty containers must be decontaminated before returning for recycling. Section 14 - TRANSPORT INFORMATION IATA Shipping Name: OXIDIZING SOLID, NOS Hazard Class: 5.1 UN Number: 1479 Packing Group: II IMO Shipping Name: OXIDIZING SOLID, NOS Hazard Class: 5.1 UN Number: 1479 Packing Group: II RID/ADR Shipping Name: OXIDIZING SOLID, NOS Hazard Class: 5.1 UN Number: 1479 Packing group: II Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: O C Risk Phrases: R 34 Causes burns. R 8 Contact with combustible material may cause fire. Safety Phrases: S 26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 28 After contact with skin, wash immediately with... WGK (Water Danger/Protection) CAS# 7782-68-5: 1 Canada CAS# 7782-68-5 is listed on Canada's DSL List. CAS# 7782-68-5 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 7782-68-5 is listed on the TSCA inventory. SECTION 16 - ADDITIONAL INFORMATION N/A |
| Symbol |


GHS03, GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H272-H314 |
| Precautionary Statements | P220-P280-P305 + P351 + P338-P310 |
| Hazard Codes | C:Corrosive |
| Risk Phrases | R34;R8 |
| Safety Phrases | S17-S26-S36/37/39-S45 |
| RIDADR | UN 3085 |
| WGK Germany | 3 |
| Packaging Group | II |
| Hazard Class | 5.1 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
|
Carbonic anhydrase inhibitors. Inhibition of isozymes I, II, IV, V, and IX with anions isosteric and isoelectronic with sulfate, nitrate, and carbonate.
Bioorg. Med. Chem. Lett. 15 , 567-71, (2005) The inhibition of five human carbonic anhydrase (hCA, EC 4.2.1.1) isozymes; the cytosolic hCA I and II, the membrane-bound hCA IV, the mitochondrial hCA V, and the tumor-associated, transmembrane hCA ... |
|
|
Multiwalled carbon nanotubes dispersed in carminic acid for the development of catalase based biosensor for selective amperometric determination of H(2)O(2) and iodate.
Biosens. Bioelectron. 29(1) , 151-8, (2011) We report the preparation of stable dispersion of multiwalled carbon nanotubes (MWCNTs) using carminic acid (CA) as a dispersing agent. The transmission electron microscopy (TEM), scanning electron mi... |
|
|
[Correlation between ERPF and retention function on 131I-OIH renogram].
Kaku Igaku. 21(10) , 1285-92, (1984)
|
| MFCD00011348 |
| acid iodate |
| Iodic acid |
| trioxoiodic(V) acid |
| Iodsaeure |
| Iodic(V) acid |
| hydrogen trioxoiodate |
| Monoiodic acid |
| hydroxidodioxidoiodine |
| EINECS 231-962-1 |
 CAS#:7732-18-5
CAS#:7732-18-5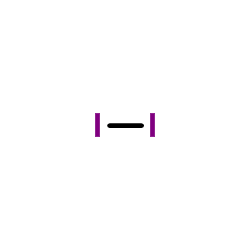 CAS#:7553-56-2
CAS#:7553-56-2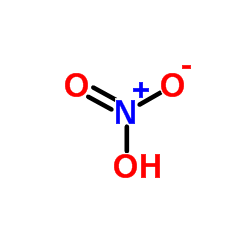 CAS#:7697-37-2
CAS#:7697-37-2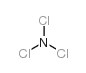 CAS#:10025-85-1
CAS#:10025-85-1 CAS#:7790-93-4
CAS#:7790-93-4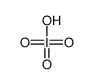 CAS#:13444-71-8
CAS#:13444-71-8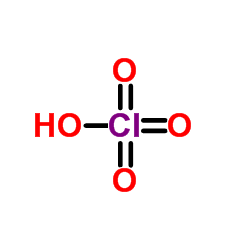 CAS#:7601-90-3
CAS#:7601-90-3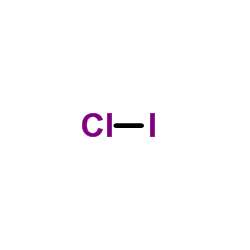 CAS#:7790-99-0
CAS#:7790-99-0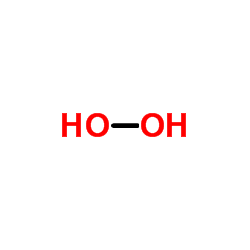 CAS#:7722-84-1
CAS#:7722-84-1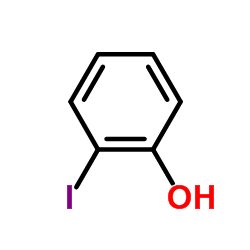 CAS#:533-58-4
CAS#:533-58-4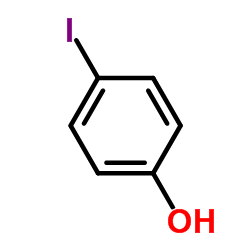 CAS#:540-38-5
CAS#:540-38-5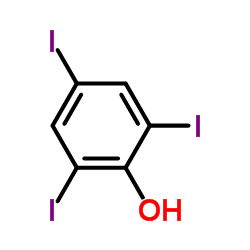 CAS#:609-23-4
CAS#:609-23-4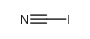 CAS#:506-78-5
CAS#:506-78-5 CAS#:14808-79-8
CAS#:14808-79-8 CAS#:7664-93-9
CAS#:7664-93-9 CAS#:7446-08-4
CAS#:7446-08-4 CAS#:13598-36-2
CAS#:13598-36-2 CAS#:10034-85-2
CAS#:10034-85-2
