Bisantrene
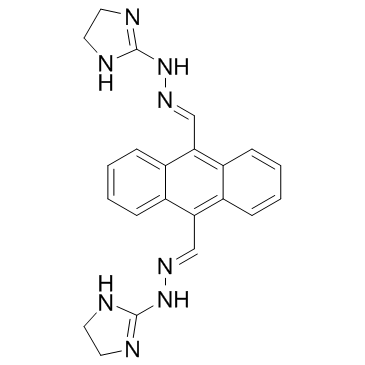
Bisantrene structure
|
Common Name | Bisantrene | ||
|---|---|---|---|---|
| CAS Number | 78186-34-2 | Molecular Weight | 398.46400 | |
| Density | 1.41g/cm3 | Boiling Point | 646.3ºC at 760 mmHg | |
| Molecular Formula | C22H22N8 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 344.7ºC | |
| Symbol |


GHS07, GHS09 |
Signal Word | Warning | |
Use of BisantreneBisantrene is a highly effective antitumor drug, targets eukaryotic type II topoisomerases. |
| Name | Bisantrene |
|---|---|
| Synonym | More Synonyms |
| Description | Bisantrene is a highly effective antitumor drug, targets eukaryotic type II topoisomerases. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase II |
| In Vivo | Bisantrene shows an outstanding ability to form a complex with DNA. Bisantrene exhibits the most effective binding (even neglecting electrostatic contacts), followed by the 9-substituted compounds and finally by 1-IHA. Bisantrene congeners retained a remarkable capacity for binding to the single-stranded structure. In comparison with the Ki values found for double-stranded DNA, 9-IHA shows a 2-fold increase, 1-IHA maintains the same values, and aza-9-IHA exhibits a modest reduction. On the other hand, Bisantrene, although undergoing a 6-fold reduction in Ki, still exhibits an affinity constant of the order of 106 M-1. Bisantrene promots DNase I cleavage at oligopurine-oligopyrimidine tracts; conversely, it slightly reduces the cleavage activity at alternating purine-pyrimidine sequences[1]. Bisantrene is an active new drug in the treatment of metastatic breast cancer. Bisantrene is an inhibitor of [3H]uridine incorporation into RNA and [3H]thymidine incorporation into DNA[2]. |
| Kinase Assay | Measurements are carried out at 25°C in ETN buffer (1 mM EDTA, 10 mM Tris, pH 7.0, with NaCl to obtain the desired ionic strength). Binding is monitored spectrophotometrically or fluorometrically, in the ligand absorption or emission region, respectively, after addition of scalar amounts of DNA to a freshly prepared drug solution. To avoid large systematic inaccuracies resulting from experimental errors in extinction coefficients or fluorescence quantum yield, the range of bound drug fractions is 0.15-0.85. Data are evaluated. Spectroscopic measurements are made with a Perkin-Elmer Lambda 5 apparatus and a MPF66 fluorometer, both equipped with a Haake F3-C thermostat[1]. |
| References |
| Density | 1.41g/cm3 |
|---|---|
| Boiling Point | 646.3ºC at 760 mmHg |
| Molecular Formula | C22H22N8 |
| Molecular Weight | 398.46400 |
| Flash Point | 344.7ºC |
| Exact Mass | 398.19700 |
| PSA | 97.56000 |
| LogP | 2.06900 |
| Index of Refraction | 1.757 |
| Water Solubility | deionized water: soluble8mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H400 |
| Precautionary Statements | P273 |
| Hazard Codes | Xn;N |
| Risk Phrases | R22;R50/53 |
| Safety Phrases | S60;S61 |
| RIDADR | UN 2811 6.1/PG 3 |
| HS Code | 2933290090 |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
In vitro effects of bisantrene on fresh clonogenic leukemia cells: a preliminary study on 15 cases.
Haematologica 75(6) , 527-31, (1990) In vitro clonogenic assays may be useful for determining the sensitivity of leukemic cells to chemotherapeutic agents. We evaluated the antileukemic effect of Bisantrene (an anthracene derivative now ... |
|
|
Phase II trial of Bisantrene for metastatic melanoma: an Illinois cancer council study.
Med. Pediatr. Oncol. 19(2) , 126-8, (1991) Sixteen patients with metastatic melanoma entered this phase II study of the efficacy of monthly cycles of Bisantrene. Toxicity was characterized by leukopenia, resulting in the hospitalization of one... |
|
|
Retroviral transfer of the human MDR1 gene confers resistance to bisantrene-specific hematotoxicity.
Clin. Cancer Res. 2(6) , 973-80, (1996) In this work, we demonstrate a protective effect conferred by the human multidrug resistance gene (MDR1) to populations of the murine hematopoietic system against the toxic effects of bisantrene, a no... |
| 9,10-Anthracenedicarbaldehyde bis[(4,5-dihydro-1H-imidazol-2-yl)hydrazone] |
| MFCD01743164 |
| 9,10-Anthracenedicarboxaldehyde bis(2-imidazolin-2-ylhydrazone) |
| Zantrene |
| ADAH |
| BISANTRENE HCL |
| anthracene-9,10-dicarbaldehyde bis-imidazolidin-2-ylidenehydrazone |
| Orange Crush |