Lanosterin
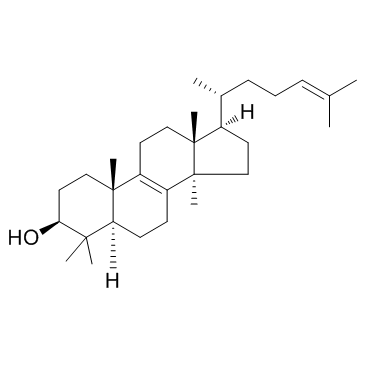
Lanosterin structure
|
Common Name | Lanosterin | ||
|---|---|---|---|---|
| CAS Number | 79-63-0 | Molecular Weight | 426.717 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 498.9±44.0 °C at 760 mmHg | |
| Molecular Formula | C30H50O | Melting Point | 137 °C | |
| MSDS | Chinese USA | Flash Point | 221.1±20.7 °C | |
Use of LanosterinLanosterol is a key triterpenoid intermediate in the biosynthesis of Cholesterol. |
| Name | lanosterol |
|---|---|
| Synonym | More Synonyms |
| Description | Lanosterol is a key triterpenoid intermediate in the biosynthesis of Cholesterol. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 498.9±44.0 °C at 760 mmHg |
| Melting Point | 137 °C |
| Molecular Formula | C30H50O |
| Molecular Weight | 426.717 |
| Flash Point | 221.1±20.7 °C |
| Exact Mass | 426.386169 |
| PSA | 20.23000 |
| LogP | 11.00 |
| Appearance of Characters | powder | white |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.530 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| RTECS | OE3360000 |
| Precursor 7 | |
|---|---|
| DownStream 3 | |
|
The mannoprotein TIR3 (CAGL0C03872g) is required for sterol uptake in Candida glabrata.
Biochim. Biophys. Acta 1851(2) , 141-51, (2015) Sterol uptake in the pathogenic fungus, Candida glabrata, occurs via the sterol transporter, CgAus1p. Azole inhibition of sterol biosynthesis can under certain circumstances be reversed by adding exog... |
|
|
Protective effect of ganodermanondiol isolated from the Lingzhi mushroom against tert-butyl hydroperoxide-induced hepatotoxicity through Nrf2-mediated antioxidant enzymes.
Food Chem. Toxicol. 53 , 317-24, (2013) Ganodermanondiol, a biologically active compound, was isolated from the Lingzhi mushroom (Ganoderma lucidum). The present study examined the protective effects of ganodermanondiol against tert-butyl h... |
|
|
FR171456 is a specific inhibitor of mammalian NSDHL and yeast Erg26p.
Nat. Commun. 6 , 8613, (2015) FR171456 is a natural product with cholesterol-lowering properties in animal models, but its molecular target is unknown, which hinders further drug development. Here we show that FR171456 specificall... |
| Lanosterol |
| Lanster |
| MFCD00021108 |
| Lanosta-8,24-dien-3-β-ol |
| Lanosterin |
| Kriptosterol |
| DUSOGEL |
| botalanbase |
| (3β)-Lanosta-8,24-dien-3-ol |
| Criptosterol |
| Lanosta-8,24-dien-3-ol, (3β)- |
| CRYPTOSTEROL |
| Kryptosterol |
| EINECS 201-214-9 |
| (3β,5α)-4,4,14-trimethyl-Cholesta-8,24-dien-3-ol |
| Lanosta-8,24-dien-3β-ol (8CI) |
| Cholesta-8,24-dien-3-ol, 4,4,14-trimethyl-, (3β,5α)- |
| Lanosta-8,24-dien-3β-ol |
| (3β,20R)-Lanosta-8,24-dien-3-ol |
| (3S,5R,10S,13R,14R,17R)-4,4,10,13,14-Pentamethyl-17-[(2R)-6-methyl-5-hepten-2-yl]-2,3,4,5,6,7,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol |
| lanosta-8,24-dien-3-ol, (3β,20R)- |
| Kryp-tosterol |
| (3b)-Lanosta-8,24-dien-3-ol |
| ISOCHOLESTEROL |
 CAS#:2671-68-3
CAS#:2671-68-3 CAS#:14050-42-1
CAS#:14050-42-1 CAS#:469-38-5
CAS#:469-38-5![[(3S,5R,10S,13R,14R,17R)-4,4,10,13,14-pentamethyl-17-[(2R)-6-methylhept-5-en-2-yl]-2,3,5,6,7,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-3-yl] octanoate Structure](https://image.chemsrc.com/caspic/301/124770-75-8.png) CAS#:124770-75-8
CAS#:124770-75-8 CAS#:63976-67-0
CAS#:63976-67-0 CAS#:13553-26-9
CAS#:13553-26-9 CAS#:68612-49-7
CAS#:68612-49-7 CAS#:1255-26-1
CAS#:1255-26-1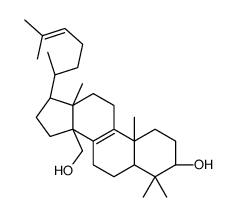 CAS#:111420-56-5
CAS#:111420-56-5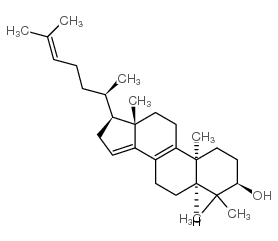 CAS#:64284-64-6
CAS#:64284-64-6
