COUMACHLOR
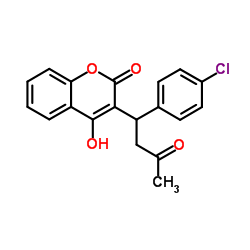
COUMACHLOR structure
|
Common Name | COUMACHLOR | ||
|---|---|---|---|---|
| CAS Number | 81-82-3 | Molecular Weight | 342.773 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 543.1±50.0 °C at 760 mmHg | |
| Molecular Formula | C19H15ClO4 | Melting Point | 168-170 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 282.3±30.1 °C | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of COUMACHLORCoumachlor is a first genereation anticoagulant rodenticide which blocks formation of prothrombin and inhibits blood coagulation causing death by internal haemorrhage. |
| Name | coumachlor |
|---|---|
| Synonym | More Synonyms |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 543.1±50.0 °C at 760 mmHg |
| Melting Point | 168-170 °C(lit.) |
| Molecular Formula | C19H15ClO4 |
| Molecular Weight | 342.773 |
| Flash Point | 282.3±30.1 °C |
| Exact Mass | 342.065887 |
| PSA | 67.51000 |
| LogP | 4.01 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.641 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H373-H412 |
| Precautionary Statements | P273 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R48/22;R52/53 |
| Safety Phrases | S37-S61 |
| RIDADR | UN 2811 6.1/PG 1 |
| WGK Germany | 2 |
| RTECS | GN4830000 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
|
~0% 
COUMACHLOR CAS#:81-82-3 
COUMACHLOR CAS#:81-82-3 |
| Literature: Advanced Synthesis and Catalysis, , vol. 355, # 13 p. 2538 - 2543 |
|
~76% 
COUMACHLOR CAS#:81-82-3 |
| Literature: Tetrahedron, , vol. 66, # 51 p. 9708 - 9713 |

COUMACHLOR CAS#:81-82-3 ~% 
COUMACHLOR CAS#:81-82-3 
COUMACHLOR CAS#:81-82-3 |
| Literature: Analytical Chemistry, , vol. 73, # 22 p. 5499 - 5508 |
|
~% 
COUMACHLOR CAS#:81-82-3 
COUMACHLOR CAS#:81-82-3 |
| Literature: Angewandte Chemie - International Edition, , vol. 42, # 40 p. 4955 - 4957 |
|
~% 
COUMACHLOR CAS#:81-82-3
Detail
|
| Literature: Tetrahedron Asymmetry, , vol. 12, # 5 p. 707 - 709 |
|
A chiral separation strategy for acidic drugs in capillary electrochromatography using both chlorinated and nonchlorinated polysaccharide-based selectors.
Electrophoresis 35(19) , 2807-18, (2014) A generic chiral separation strategy for the analysis of acidic compounds in CEC is proposed in completion of an earlier defined strategy for nonacidic compounds. The screening step of this strategy u... |
|
|
Single-step approach for fabrication of vancomycin-bonded silica monolith as chiral stationary phase.
J. Chromatogr. A. 1358 , 208-16, (2014) A vancomycin-bonded silica monolithic column for capillary electrochromatography (CEC) was prepared by a single-step in situ sol-gel approach. This sol-gel process incorporates a synthetic sol-gel pre... |
|
|
Toxicology and histopathology of some rodenticides and palatable food items combinations on the common mice Mus musculus var. albus in Egypt.
Commun. Agric. Appl. Biol. Sci. 68(4 Pt B) , 771-87, (2003) In this study the palatability tests of certain food items as attractants in the poisoned-baits for the albino mouse Mus musculus var. albus showed that the food items of treacle, maize oil, dry or we... |
| UNII:UCD8XZW42P |
| 3-(1-(4-Chlorophenyl)-3-oxobutyl)-4-hydroxy-2H-1-benzopyran-2-one (9CI) |
| MFCD00012094 |
| 2H-1-Benzopyran-2-one, 3-[1-(4-chlorophenyl)-3-oxobutyl]-4-hydroxy- |
| 3-(a-Acetonyl-p-chlorobenzyl)-4-hydroxycoumarin |
| tomorin |
| 3-[(1RS)-1-(4-chlorophenyl)-3-oxobutyl]-4-hydroxycoumarin |
| 3-(a-p-Chlorophenyl-b-acetylethyl)-4-hydroxycoumarin |
| 3-[1-(4-Chlorophenyl)-3-oxobutyl]-4-hydroxy-2H-1-benzopyran-2-one |
| Coumachlor |
| Geigy Rodenticide Exp.332 |
| FAMARIN |
| rac-3-[(1R)-1-(4-chlorophenyl)-3-oxobutyl]-4-hydroxy-2H-1-benzopyran-2-one |
| 3-[1-(4-Chlorophenyl)-3-oxobutyl]-4-hydroxy-2H-chromen-2-one |
| ratilan |
| P-CHLOROWARFARIN |
| cumachloor |
| kumachlor |
| (±)-3-(1-(p-Chlorophenyl)-3-oxobutyl)-4-hydroxycoumarin |
| EINECS 201-378-1 |
| (±)-Coumachlor |