Quinapril hydrochloride
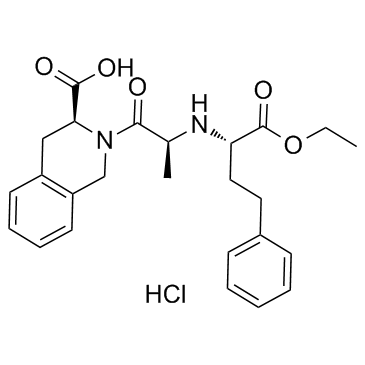
Quinapril hydrochloride structure
|
Common Name | Quinapril hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 82586-55-8 | Molecular Weight | 474.977 | |
| Density | N/A | Boiling Point | 662ºC at 760 mmHg | |
| Molecular Formula | C25H31ClN2O5 | Melting Point | 120-130ºC | |
| MSDS | USA | Flash Point | 354.1ºC | |
Use of Quinapril hydrochlorideQuinapril is a prodrug that belongs to the angiotensin-converting enzyme (ACE) inhibitor class of medications.Target: ACEQuinapril is an angiotensin-converting enzyme inhibitor (ACE inhibitor) used in the treatment of hypertension and congestive heart failure. Quinapril is rapidly de-esterified after absorption to quinaprilat (the active diacid metabolite), a potent angiotensin converting enzyme (ACE) inhibitor. Quinapril is now firmly established as an effective and well tolerated ACE inhibitor for the treatment of patients with hypertension and congestive heart failure. Quinapril 40 mg/day also significantly reduced the incidence of ischaemic events in patients undergoing CABG in one study [1, 2]. An overview of 32 clinical trials of ACE inhibitors in heart failure showed that no significant heterogeneity in mortality was found among enalapril, ramipril, quinapril, captopril, lisinopril, benazepril, perindopril and cilazapril. Initiation of therapy with captopril, ramipril, and trandolapril at least 3 days after an acute MI resulted in all-cause mortality risk reductions of 18 to 27% [3]. |
| Name | quinapril hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Quinapril is a prodrug that belongs to the angiotensin-converting enzyme (ACE) inhibitor class of medications.Target: ACEQuinapril is an angiotensin-converting enzyme inhibitor (ACE inhibitor) used in the treatment of hypertension and congestive heart failure. Quinapril is rapidly de-esterified after absorption to quinaprilat (the active diacid metabolite), a potent angiotensin converting enzyme (ACE) inhibitor. Quinapril is now firmly established as an effective and well tolerated ACE inhibitor for the treatment of patients with hypertension and congestive heart failure. Quinapril 40 mg/day also significantly reduced the incidence of ischaemic events in patients undergoing CABG in one study [1, 2]. An overview of 32 clinical trials of ACE inhibitors in heart failure showed that no significant heterogeneity in mortality was found among enalapril, ramipril, quinapril, captopril, lisinopril, benazepril, perindopril and cilazapril. Initiation of therapy with captopril, ramipril, and trandolapril at least 3 days after an acute MI resulted in all-cause mortality risk reductions of 18 to 27% [3]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 662ºC at 760 mmHg |
|---|---|
| Melting Point | 120-130ºC |
| Molecular Formula | C25H31ClN2O5 |
| Molecular Weight | 474.977 |
| Flash Point | 354.1ºC |
| Exact Mass | 474.192139 |
| PSA | 95.94000 |
| LogP | 3.69790 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
Quinapril hydro... CAS#:82586-55-8 |
| Literature: WO2004/54980 A1, ; Page 25;13;16 ; |
|
~% 
Quinapril hydro... CAS#:82586-55-8 |
| Literature: US2004/192613 A1, ; Page/Page column 5 ; |
|
Determination of losartan potassium, quinapril hydrochloride and hydrochlorothiazide in pharmaceutical preparations using derivative spectrophotometry and chromatographic-densitometric method.
Acta Pol. Pharm. 70(6) , 967-76, (2013) Two methods, spectrophotometric and chromatographic-densitometric ones, were developed for determination of losartan potassium, quinapril hydrochloride and hydrochlorothiazide in pharmaceutical prepar... |
|
|
Hepatic, intestinal, renal, and plasma hydrolysis of prodrugs in human, cynomolgus monkey, dog, and rat: implications for in vitro-in vivo extrapolation of clearance of prodrugs.
Drug Metab. Dispos. 42(9) , 1522-31, (2014) Hydrolysis plays an important role in metabolic activation of prodrugs. In the current study, species and in vitro system differences in hepatic and extrahepatic hydrolysis were investigated for 11 pr... |
|
|
The impact of lipoic acid on endothelial function and proteinuria in quinapril-treated diabetic patients with stage I hypertension: results from the QUALITY study.
J. Cardiovasc. Pharmacol. Ther. 17(2) , 139-45, (2012) We sought to determine whether a combination of angiotensin-converting enzyme inhibitors (ACEIs) and the nutraceutical α-lipoic acid (ALA) regulates blood pressure, endothelial function, and proteinur... |
| (3S)-2-[(2S)-2-{[(1S)-1-(Ethoxycarbonyl)-3-phenylpropyl]amino}propanoyl]-1,2,3,4-tetrahydroisochinolin-3-carbonsäurehydrochlorid |
| (3S)-2-{N-[(2S)-1-Ethoxy-1-oxo-4-phenyl-2-butanyl]-L-alanyl}-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic acid hydrochloride (1:1) |
| (3S)-2-[(2S)-2-{[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino}propanoyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid hydrochloride |
| Acuitel |
| 3-Isoquinolinecarboxylic acid, 2-[(2S)-2-[[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydro-, (3S)-, hydrochloride (1:1) |
| ccupril |
| Acequin |
| quinapril HCl |
| 2-<<1-ethoxycarbonyl)-3-phenylpropyl>amino>-1-oxopropyl>-1,2,3,4-tetrahydro-3-isoquinoline carboxylic acid monohydrochloride |
| Conan |
| ACCUPRIN |
| (3S)-2-{N-[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid hydrochloride |
| 3-isoquinolinecarboxylic acid, 2-[(2S)-2-[[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydro-, (3S)-, monohydrochloride |
| Quinapril hydrochloride |
| Acupril |
| (3S)-2-{N-[(2S)-1-Ethoxy-1-oxo-4-phenylbutan-2-yl]-L-alanyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid hydrochloride (1:1) |
| CI-906 |
| Acuprel |
| MFCD00889215 |
| ACCUPRO |
| Korec |
| acide (3S)-2-[(2S)-2-{[(1S)-1-(éthoxycarbonyl)-3-phénylpropyl]amino}propanoyl]-1,2,3,4-tétrahydroisoquinoléine-3-carboxylique chlorhydrate |
| Quinapril (hydrochloride) |
| QuinaprilHydrochloride |
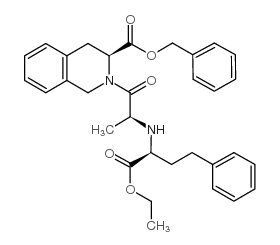
![(1S,2S,3S)-2-[2-(1-ethoxycarbonyl-3-phenyl-propylamino)-propionyl] -1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid tert-butyl ester structure](https://image.chemsrc.com/caspic/369/82586-56-9.png)
 CAS#:85441-61-8
CAS#:85441-61-8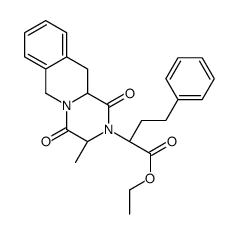 CAS#:103733-49-9
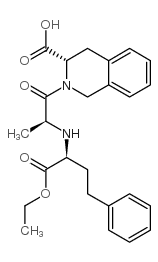
CAS#:103733-49-9 CAS#:82768-85-2
CAS#:82768-85-2![Ethyl (3S)-2-{N-[(2S)-1-ethoxy-1-oxo-4-phenyl-2-butanyl]-L-alanyl }-1,2,3,4-tetrahydro-3-isoquinolinecarboxylate structure](https://image.chemsrc.com/caspic/334/103733-35-3.png) CAS#:103733-35-3
CAS#:103733-35-3
