(S)-Benzyl (1-(1H-benzo[d][1,2,3]triazol-1-yl)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamate
![(S)-Benzyl (1-(1H-benzo[d][1,2,3]triazol-1-yl)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamate Structure](https://image.chemsrc.com/caspic/472/850232-59-6.png)
(S)-Benzyl (1-(1H-benzo[d][1,2,3]triazol-1-yl)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamate structure
|
Common Name | (S)-Benzyl (1-(1H-benzo[d][1,2,3]triazol-1-yl)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamate | ||
|---|---|---|---|---|
| CAS Number | 850232-59-6 | Molecular Weight | 439.46600 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C25H21N5O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | Cbz-L-Cys(Bz)-Bt |
|---|---|
| Synonym | More Synonyms |
| Molecular Formula | C25H21N5O3 |
|---|---|
| Molecular Weight | 439.46600 |
| Exact Mass | 439.16400 |
| PSA | 101.90000 |
| LogP | 4.48140 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| RIDADR | NONH for all modes of transport |
| Precursor 0 | |
|---|---|
| DownStream 2 | |
|
Benzotriazole-mediated syntheses of depsipeptides and oligoesters.
J. Org. Chem. 76 , 4884-4893, (2011) Reactions of O-Pg(α-hydroxyacyl)benzotriazoles with (a) unprotected α-hydroxycarboxylic acids, (b) amino acids, and (c) amines afforded, respectively, chirally pure (a) oligoesters, (b) depsidipeptide... |
|
|
Efficient preparation of aminoxyacyl amides, aminoxy hybrid peptides, and alpha-aminoxy peptides.
J. Org. Chem. 74th ed.,, 8690-8694, (2009) N-(Pg-alpha-aminoxy acids) 1a-g are converted to N-(Pg-alpha-aminoxyacyl)benzotriazoles 2a-g, which react under mild conditions with amines, alpha-amino acids/alpha-dipeptides, and alpha-aminoxy acids... |
|
|
Abdelmajeid, A.; Tala, S. R.; Amine, M. S.; Katritzky, A. R.
Synthesis , 2995-3005, (2011)
|
| N-Z-L-Trp-Bt |
| benzyl N-[(1S)-2-(1H-1,2,3-benzotriazol-1-yl)-1-(1H-indol-3-ylmethyl)-2-oxoethyl]carbamate |
| Z-TRP-BT |
| Cbz-L-Trp-Bt |
| Z-L-Trp-Bt |
| (S)-benzyl [1-(1H-benzo[d][1,2,3]triazol-1-yl)-3-(benzylthio)-1-oxopropan-2-yl]carbamate |
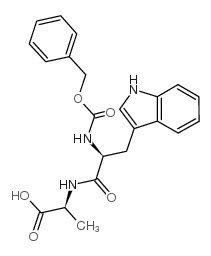 CAS#:17388-71-5
CAS#:17388-71-5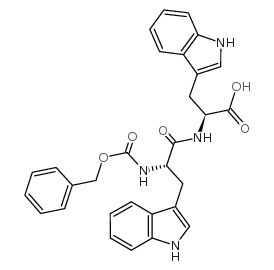 CAS#:57850-17-6
CAS#:57850-17-6