1-(3,5-BIS(TRIFLUOROMETHYL)PHENYL)-3-((1R)-(6-METHOXYQUINOLIN-4-YL)((2R,4S,5R)-5-VINYLQUINUCLIDIN-2-YL)METHYL)THIOUREA
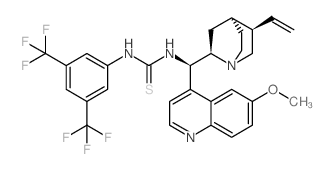
1-(3,5-BIS(TRIFLUOROMETHYL)PHENYL)-3-((1R)-(6-METHOXYQUINOLIN-4-YL)((2R,4S,5R)-5-VINYLQUINUCLIDIN-2-YL)METHYL)THIOUREA structure
|
Common Name | 1-(3,5-BIS(TRIFLUOROMETHYL)PHENYL)-3-((1R)-(6-METHOXYQUINOLIN-4-YL)((2R,4S,5R)-5-VINYLQUINUCLIDIN-2-YL)METHYL)THIOUREA | ||
|---|---|---|---|---|
| CAS Number | 852913-25-8 | Molecular Weight | 594.61400 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C29H28F6N4OS | Melting Point | 150°C | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS06 |
Signal Word | Danger | |
| Name | 1-[3,5-bis(trifluoromethyl)phenyl]-3-[(R)-[(2R,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methyl]thiourea |
|---|---|
| Synonym | More Synonyms |
| Melting Point | 150°C |
|---|---|
| Molecular Formula | C29H28F6N4OS |
| Molecular Weight | 594.61400 |
| Exact Mass | 594.18900 |
| PSA | 81.51000 |
| LogP | 7.60690 |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| RIDADR | UN 2811 6.1 / PGIII |
|
~61% 
1-(3,5-BIS(TRIF... CAS#:852913-25-8 |
| Literature: Berkessel, Albrecht; Mukherjee, Santanu; Mueller, Thomas N.; Cleemann, Felix; Roland, Katrin; Brandenburg, Marc; Neudoerfl, Joerg-M.; Lex, Johann Organic and Biomolecular Chemistry, 2006 , vol. 4, # 23 p. 4319 - 4330 |
|
~% 
1-(3,5-BIS(TRIF... CAS#:852913-25-8 |
| Literature: Asano, Keisuke; Matsubara, Seijiro Journal of the American Chemical Society, 2011 , vol. 133, # 42 p. 16711 - 16713 |
|
~% 
1-(3,5-BIS(TRIF... CAS#:852913-25-8 |
| Literature: Asano, Keisuke; Matsubara, Seijiro Journal of the American Chemical Society, 2011 , vol. 133, # 42 p. 16711 - 16713 |
|
Urea- and thiourea-substituted cinchona alkaloid derivatives as highly efficient bifunctional organocatalysts for the asymmetric addition of malonate to nitroalkenes: inversion of configuration at C9 dramatically improves catalyst performance.
Angew. Chem. Int. Ed. Engl. 44 , 6367-6370, (2005)
|
|
|
Highly enantioselective conjugate addition of nitromethane to chalcones using bifunctional cinchona organocatalysts.
Org. Lett. 7 , 1967-1969, (2005) Cinchona alkaloid-derived chiral bifunctional thiourea organocatalysts were synthesized and applied in the Michael addition between nitromethane and chalcones with high ee and chemical yields. |
| 1-[3,5-bis(trifluoromethyl)phenyl]-3-[(R)-(6-methoxyquinolin-4-yl)-(8-vinylquinuclidin-2-yl)methyl]thiourea |
| 1-[3,5-BIS(TRIFLUOROMETHYL)PHENYL]PROPANOL-1 MIN. |
| 1-[3,5-bis(trifluoromethyl)phenyl]-1-propanol |
| 1-[3,5-BIS(TRIFLUOROMETHYL)PHENYL]PROPANOL-1 |

![4-[(R)-azido[(2R,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]methyl]-6-methoxyquinoline structure](https://image.chemsrc.com/caspic/400/866140-03-6.png)

