Ivacaftor (VX-770)
Modify Date: 2024-01-02 23:43:51
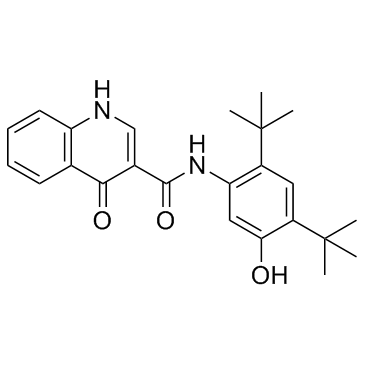
Ivacaftor (VX-770) structure
|
Common Name | Ivacaftor (VX-770) | ||
|---|---|---|---|---|
| CAS Number | 873054-44-5 | Molecular Weight | 392.491 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 550.5±50.0 °C at 760 mmHg | |
| Molecular Formula | C24H28N2O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 286.7±30.1 °C | |
Use of Ivacaftor (VX-770)Ivacaftor is a potent and orally bioavailable CFTR potentiator, targeting G551D-CFTR and F508del-CFTR with EC50s of 100 nM and 25 nM, respectively. |
| Name | ivacaftor |
|---|---|
| Synonym | More Synonyms |
| Description | Ivacaftor is a potent and orally bioavailable CFTR potentiator, targeting G551D-CFTR and F508del-CFTR with EC50s of 100 nM and 25 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
EC50: 100 nM (G551D-CFTR), 25 nM (F508del-CFTR)[1] |
| In Vitro | Ivacaftor (10 µM) increases the PC secretion activity by 3-fold for ABCB4-G535D, 13.7-fold for ABCB4-G536R, 6.7-fold for ABCB4-S1076C, 9.4-fold for ABCB4-S1176L, and 5.7-fold for ABCB4-G1178S. Ivacaftor corrects the functional defect of ABCB4 mutants[1]. Ivacaftor (10 μM) significantly increases CFTR activity in W1282X-expressing cells compared to R1162X CFTR cells[2]. Ivacaftor shows no significant activity against 160 targets tested including the GABAA benzodiazepine receptor. Ivacaftor increases the chloride secretion with an EC50 of 0.236 ± 0.200 μM, a 10-fold shift in potency compared to the F508del HBEs[3]. In recombinant cells, VX-770 increases CFTR channel open probability (Po) in both the F508del processing mutation and the G551D gating mutation. VX-770 increases forskolin-stimulated IT in temperature-corrected F508del-FRT cells by appr 6-fold with an EC50 of 25 nM[4]. |
| In Vivo | Ivacaftor (1-200 mg/kg, p.o.) exhibits good oral bioavailability in rat[3]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 550.5±50.0 °C at 760 mmHg |
| Molecular Formula | C24H28N2O3 |
| Molecular Weight | 392.491 |
| Flash Point | 286.7±30.1 °C |
| Exact Mass | 392.209991 |
| PSA | 85.68000 |
| LogP | 6.34 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.606 |
| Storage condition | 2~8℃ |
| HS Code | 29333990 |
|---|
| Precursor 9 | |
|---|---|
| DownStream 2 | |
| 3-Quinolinecarboxamide, N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxo- |
| Ivacaftor |
| [14C]-Ivacaftor |
| Kalydeco |
| N-(2,4-ditert-butyl-5-hydroxyphenyl)-4-oxo-1H-quinoline-3-carboxamide |
| MFCD17171361 |
| VX770 |
| UNII-1Y740ILL1Z |
| N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxaraide |
| N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline-3-carboxamide |
| N-[5-Hydroxy-2,4-bis(2-methyl-2-propanyl)phenyl]-4-oxo-1,4-dihydro-3-quinolinecarboxamide |
| N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide |
| N-[2,4-Bis(tert-butyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxo-3-quinolinecarboxamide |
| VX-770 |
 CAS#:873055-58-4
CAS#:873055-58-4 CAS#:1182822-31-6
CAS#:1182822-31-6 CAS#:13721-01-2
CAS#:13721-01-2![Carbonic acid 5-[[(1,4-dihydro-4-oxo-3-quinolinyl)carbonyl]amino]-2,4-bis(1,1-dimethylethyl)phenyl methyl ester Structure](https://image.chemsrc.com/caspic/441/1246213-45-5.png) CAS#:1246213-45-5
CAS#:1246213-45-5 CAS#:96-76-4
CAS#:96-76-4 CAS#:873055-54-0
CAS#:873055-54-0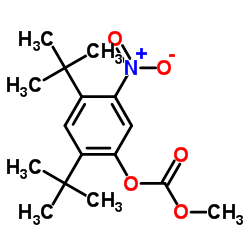 CAS#:873055-55-1
CAS#:873055-55-1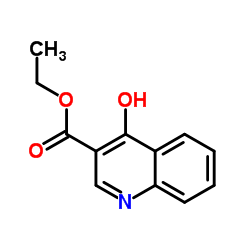 CAS#:52980-28-6
CAS#:52980-28-6![Propanedioic acid,2-[(phenylamino)methylene]-, 1,3-diethyl ester Structure](https://image.chemsrc.com/caspic/275/54535-22-7.png) CAS#:54535-22-7
CAS#:54535-22-7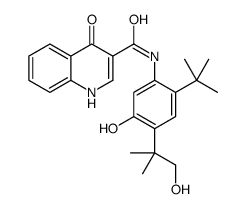 CAS#:1246213-23-9
CAS#:1246213-23-9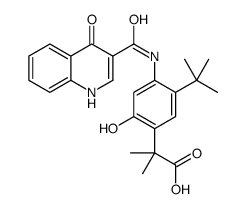 CAS#:1246213-24-0
CAS#:1246213-24-0
