1,3-Dimethyluracil
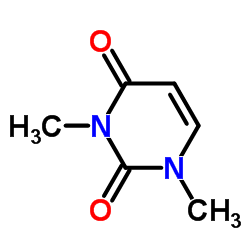
1,3-Dimethyluracil structure
|
Common Name | 1,3-Dimethyluracil | ||
|---|---|---|---|---|
| CAS Number | 874-14-6 | Molecular Weight | 140.140 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 208.4±23.0 °C at 760 mmHg | |
| Molecular Formula | C6H8N2O2 | Melting Point | 119-122 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 84.3±15.0 °C | |
Use of 1,3-Dimethyluracil1,3-Dimethyluracil is a pyrimidone derives from a uracil. 1,3-Dimethyluracil found occasionally in human urine. 1,3-Dimethyluracil shows inhibition activity against hCA I and hCA II (human carbonic anhydrase) with Ki of 316.2 μM and 166.4 μM, respectively[1][2]. |
| Name | 1,3-Dimethyluracil |
|---|---|
| Synonym | More Synonyms |
| Description | 1,3-Dimethyluracil is a pyrimidone derives from a uracil. 1,3-Dimethyluracil found occasionally in human urine. 1,3-Dimethyluracil shows inhibition activity against hCA I and hCA II (human carbonic anhydrase) with Ki of 316.2 μM and 166.4 μM, respectively[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 208.4±23.0 °C at 760 mmHg |
| Melting Point | 119-122 °C(lit.) |
| Molecular Formula | C6H8N2O2 |
| Molecular Weight | 140.140 |
| Flash Point | 84.3±15.0 °C |
| Exact Mass | 140.058578 |
| PSA | 44.00000 |
| LogP | 0.40 |
| Vapour Pressure | 0.2±0.4 mmHg at 25°C |
| Index of Refraction | 1.519 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36 |
| Safety Phrases | S22-S24/25-S39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933599090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Excited state structures and decay dynamics of 1,3-dimethyluracils in solutions: resonance Raman and quantum mechanical calculation study.
J. Phys. Chem. B 117(39) , 11660-9, (2013) The resonance Raman spectroscopic study of the excited state structural dynamics of 1,3-dimethyluracil (DMU), 5-bromo-1,3-dimethyluracil (5BrDMU), uracil, and thymine in water and acetonitrile were re... |
|
|
Ionization of dimethyluracil dimers leads to facile proton transfer in the absence of hydrogen bonds.
Nature Chemistry 4(4) , 323-9, (2012) Proton transfer is ubiquitous in chemistry and biology, occurring, for example, in proteins, enzyme reactions and across proton channels and pumps. However, it has always been described in the context... |
|
|
The SO4(.-)-induced chain reaction of 1,3-dimethyluracil with peroxodisulphate.
Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 51(3) , 441-53, (1987) The sulphate radical SO4(.-) reacts with 1,3-dimethyluracil (1,3-DMU) (k = 5 X 10(9) dm3 mol-1 s-1) thereby forming with greater than or equal to 90 per cent yield the 1,3-DMU C(5)-OH adduct radical 4... |
| N1,N3-Dimethyluracil |
| 1,3-dimethylpyrimidine-2,4-dione |
| 2,4(1H,3H)-Pyrimidinedione, 1,3-dimethyl- |
| 1,3-Dimethyl-2,4(1H,3H)-pyrimidinedione |
| MFCD00038065 |
| 1,3-dimethyluracil |
| Uracil, 1,3-dimethyl- |
| 1,3-dimethylpyrimidine-2,4(1H,3H)-dione |
| EINECS 212-856-4 |
 CAS#:66-22-8
CAS#:66-22-8 CAS#:77-78-1
CAS#:77-78-1 CAS#:7033-39-8
CAS#:7033-39-8 CAS#:616-38-6
CAS#:616-38-6 CAS#:74-88-4
CAS#:74-88-4 CAS#:74-83-9
CAS#:74-83-9 CAS#:83174-90-7
CAS#:83174-90-7 CAS#:10504-60-6
CAS#:10504-60-6 CAS#:32894-07-8
CAS#:32894-07-8![Ethyl 4,7-dihydro-7-oxopyrazolo[1,5-a]pyrimidine-3-carboxylate structure](https://image.chemsrc.com/caspic/232/104556-86-7.png) CAS#:104556-86-7
CAS#:104556-86-7![3-methyl-4h-pyrazolo[1,5-a]pyrimidin-7-one structure](https://image.chemsrc.com/caspic/270/104556-85-6.png) CAS#:104556-85-6
CAS#:104556-85-6 CAS#:35441-10-2
CAS#:35441-10-2 CAS#:35441-11-3
CAS#:35441-11-3 CAS#:41613-26-7
CAS#:41613-26-7 CAS#:40738-83-8
CAS#:40738-83-8 CAS#:96-31-1
CAS#:96-31-1![8,10-dimethyl-2,8,10-triazabicyclo[4.4.0]deca-4,11-diene-3,7,9-trione structure](https://image.chemsrc.com/caspic/179/57821-20-2.png) CAS#:57821-20-2
CAS#:57821-20-2 CAS#:58-55-9
CAS#:58-55-9
