1-Naphthalenol
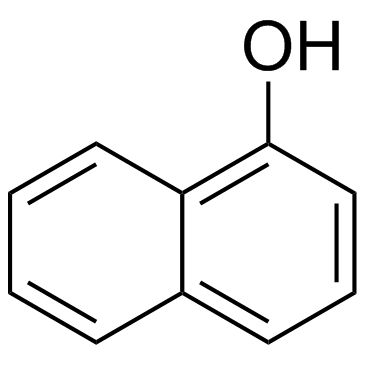
1-Naphthalenol structure
|
Common Name | 1-Naphthalenol | ||
|---|---|---|---|---|
| CAS Number | 90-15-3 | Molecular Weight | 144.170 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 288.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C10H8O | Melting Point | 94-98ºC | |
| MSDS | Chinese USA | Flash Point | 144.0±10.6 °C | |
| Symbol |



GHS05, GHS06, GHS09 |
Signal Word | Danger | |
Use of 1-Naphthalenol1-naphthol is an excited state proton transfer (ESPT) fluorescent molecular probe. |
| Name | 1-naphthol |
|---|---|
| Synonym | More Synonyms |
| Description | 1-naphthol is an excited state proton transfer (ESPT) fluorescent molecular probe. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 288.0±0.0 °C at 760 mmHg |
| Melting Point | 94-98ºC |
| Molecular Formula | C10H8O |
| Molecular Weight | 144.170 |
| Flash Point | 144.0±10.6 °C |
| Exact Mass | 144.057510 |
| PSA | 20.23000 |
| LogP | 2.71 |
| Vapour density | 4.5 (120 °C, vs air) |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.678 |
| Storage condition | Store in dark! |
| Stability | Stable, but air and light sensitive - store under inert gas. Incompatible with strong bases, strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS05, GHS06, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H311-H315-H318-H335-H411 |
| Precautionary Statements | P273-P280-P302 + P352 + P312-P305 + P351 + P338 + P310-P391 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R21/22;R37/38;R41 |
| Safety Phrases | S2-S22-S26-S37/39 |
| RIDADR | UN 1759 |
| WGK Germany | 1 |
| RTECS | QL2800000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2907151000 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2907151000 |
|---|---|
| Summary | HS:2907151000 naphthalen-2-ol VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts.
Nat. Commun. 5 , 5215, (2014) The hormone calcitonin (CT) is primarily known for its pharmacologic action as an inhibitor of bone resorption, yet CT-deficient mice display increased bone formation. These findings raised the questi... |
|
|
Rosmarinic acid attenuates hepatic ischemia and reperfusion injury in rats.
Food Chem. Toxicol. 74 , 270-8, (2015) Rosmarinic acid (RosmA) demonstrates antioxidant and anti-inflammatory properties. We investigated the effect of RosmA on liver ischemia/reperfusion injury. Rats were submitted to 60 min of ischemia p... |
|
|
Local administration of stromal cell-derived factor-1 promotes stem cell recruitment and bone regeneration in a rat periodontal bone defect model.
Mater. Sci. Eng. C. Mater. Biol. Appl. 53 , 83-94, (2015) Stromal cell-derived factor-1 (SDF-1) recruits adult stem/progenitor cells via its specific receptor, C-X-C motif receptor 4 (CXCR4), to promote heart, kidney and tendon regeneration, but little is kn... |
| Ursol ERN |
| 1-Naphthol |
| 1-Naphthalenol |
| Molisch's reagent |
| nakotrb |
| [14C]-1-Naphthol |
| L66J BQ |
| Zoba ERN |
| α-Naphthol |
| 1-hydroxynaphthalene |
| EINECS 201-969-4 |
| 1-naphtol |
| MFCD00003930 |
| α-Hydroxynaphthalene |
| naphthalen-1-ol |
| furro er |
 CAS#:6202-48-8
CAS#:6202-48-8 CAS#:90-11-9
CAS#:90-11-9 CAS#:573-57-9
CAS#:573-57-9 CAS#:90-14-2
CAS#:90-14-2 CAS#:90-13-1
CAS#:90-13-1 CAS#:13922-41-3
CAS#:13922-41-3 CAS#:5328-01-8
CAS#:5328-01-8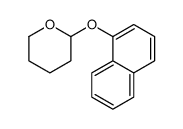 CAS#:80116-05-8
CAS#:80116-05-8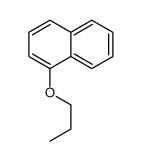 CAS#:20009-26-1
CAS#:20009-26-1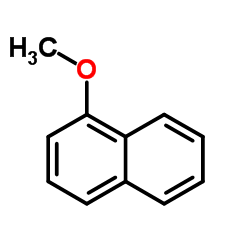 CAS#:2216-69-5
CAS#:2216-69-5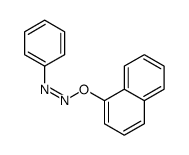 CAS#:109057-65-0
CAS#:109057-65-0 CAS#:3218-36-8
CAS#:3218-36-8 CAS#:3375-23-3
CAS#:3375-23-3 CAS#:3651-02-3
CAS#:3651-02-3 CAS#:65-85-0
CAS#:65-85-0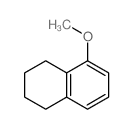 CAS#:1008-19-1
CAS#:1008-19-1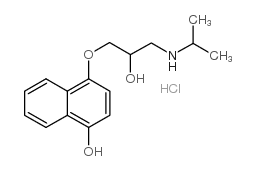 CAS#:10476-53-6
CAS#:10476-53-6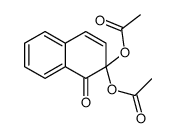 CAS#:100622-13-7
CAS#:100622-13-7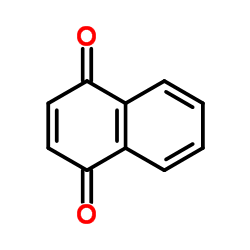 CAS#:130-15-4
CAS#:130-15-4 CAS#:107727-85-5
CAS#:107727-85-5
