Clostripain from Clostridium histolyticum
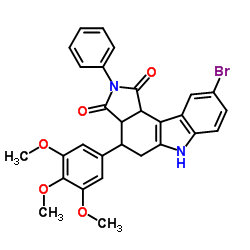
Clostripain from Clostridium histolyticum structure
|
Common Name | Clostripain from Clostridium histolyticum | ||
|---|---|---|---|---|
| CAS Number | 9028-00-6 | Molecular Weight | 561.423 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 751.6±60.0 °C at 760 mmHg | |
| Molecular Formula | C29H25BrN2O5 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 408.4±32.9 °C | |
Use of Clostripain from Clostridium histolyticumClostripain (Clostridiopeptidase B) is a proteolytic enzyme isolated from Clostridium histolyticum with esterase, amidase and protease activities and is a highly specific carboxypeptide targeting arginine key protease[1]. |
| Name | Clostripain |
|---|---|
| Synonym | More Synonyms |
| Description | Clostripain (Clostridiopeptidase B) is a proteolytic enzyme isolated from Clostridium histolyticum with esterase, amidase and protease activities and is a highly specific carboxypeptide targeting arginine key protease[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 751.6±60.0 °C at 760 mmHg |
| Molecular Formula | C29H25BrN2O5 |
| Molecular Weight | 561.423 |
| Flash Point | 408.4±32.9 °C |
| Exact Mass | 560.094666 |
| LogP | 4.55 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.669 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
|
Determination of the cytotoxic effect of Clostridium histolyticum culture supernatant on HeLa cells in the presence of protease inhibitors.
FEMS Immunol. Med. Microbiol. 45(2) , 137-42, (2005) Clostridium histolyticum culture supernatant contains numerous enzymes, which exert a cytotoxic effect on host cells. This includes lethal toxin, clostripain and high-potassium-sensitive toxin. Since ... |
|
|
High-level production and purification of clostripain expressed in a virulence-attenuated strain of Clostridium perfringens.
Protein Expr. Purif. 76(1) , 83-9, (2011) Clostripain (CLO) produced by Clostridium histolyticum is an arginine-specific endopeptidase with the potential for applicability to diverse medical and industrial uses. In this study, we developed an... |
|
|
Protease-mediated ligation of abiotic oligomers.
J. Am. Chem. Soc. 127(49) , 17132-3, (2005) We demonstrate that proteases can catalyze the ligation of peptidomimetic oligomers. The enzyme clostripain was used to facilitate the native ligation of N-substituted glycine oligomers, or peptoids. ... |
| MFCD00130814 |
| Pyrrolo[3,4-c]carbazole-1,3(2H,3aH)-dione, 9-bromo-4,5,6,10c-tetrahydro-2-phenyl-4-(3,4,5-trimethoxyphenyl)- |
| 9-Bromo-2-phenyl-4-(3,4,5-trimethoxyphenyl)-4,5,6,10c-tetrahydropyrrolo[3,4-c]carbazole-1,3(2H,3aH)-dione |

