esatenolol
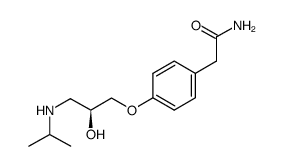
esatenolol structure
|
Common Name | esatenolol | ||
|---|---|---|---|---|
| CAS Number | 93379-54-5 | Molecular Weight | 266.33600 | |
| Density | 1.125 g/cm3 | Boiling Point | 508ºC at 760mmHg | |
| Molecular Formula | C14H22N2O3 | Melting Point | 148-152 ºC(lit.) | |
| MSDS | USA | Flash Point | 261.1ºC | |
| Name | esatenolol |
|---|---|
| Synonym | More Synonyms |
| Density | 1.125 g/cm3 |
|---|---|
| Boiling Point | 508ºC at 760mmHg |
| Melting Point | 148-152 ºC(lit.) |
| Molecular Formula | C14H22N2O3 |
| Molecular Weight | 266.33600 |
| Flash Point | 261.1ºC |
| Exact Mass | 266.16300 |
| PSA | 84.58000 |
| LogP | 1.54330 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | CY1488020 |
| HS Code | 2924299090 |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
Effects of nebivolol on endothelial gene expression during oxidative stress in human umbilical vein endothelial cells.
Mediators Inflamm. 2008 , 367590, (2008) The endothelium plays a key role in the development of atherogenesis and its inflammatory and proliferative status influences the progression of atherosclerosis. The aim of this study is to compare th... |
|
|
The effects of (+/-)-, (+)-, and (-)-atenolol, sotalol, and amosulalol on the rat left atria and portal vein.
Chirality 5 , 8-14, (1993) The effects of (+/-)-, (+)-, and (-)-atenolol, sotalol, and amosulalol alone on the rat left atria and portal vein and on the respective beta 1- and beta 2-adrenoceptor-mediated responses to isoprenal... |
| (-)-4-[2-hydroxy-3-[(1-methylethyl)amino]propoxy]benzeneacetamide |
| S-(-)-Atenolol |
| (S)-4-[2-hydroxy-3-((1-methylethyl)amino)propoxy]benzeneacetamide |
| MFCD00074918 |
| (-)-Atenolol |
| 1-Phenylbiguanide hydrochloride |
| 2-[4-[(2S)-2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl]acetamide |
| Esatenolol [INN] |
| 4-{(2S)-2-hydroxy-3-[(1-methylethyl)amino]propoxy}benzeneacetamide |
| Esatenolol |
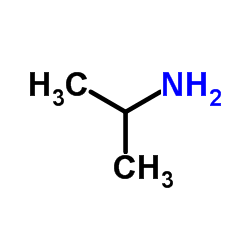 CAS#:75-31-0
CAS#:75-31-0![4-[(2S)-2-Oxiranylmethoxy]benzeneacetamide Structure](https://image.chemsrc.com/caspic/368/56715-12-9.png) CAS#:56715-12-9
CAS#:56715-12-9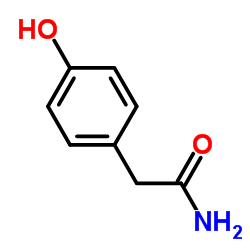 CAS#:17194-82-0
CAS#:17194-82-0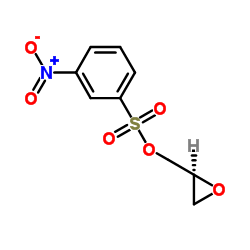 CAS#:115314-14-2
CAS#:115314-14-2 CAS#:106-89-8
CAS#:106-89-8 CAS#:156-38-7
CAS#:156-38-7 CAS#:79419-46-8
CAS#:79419-46-8