Ruxolitinib (INCB018424)
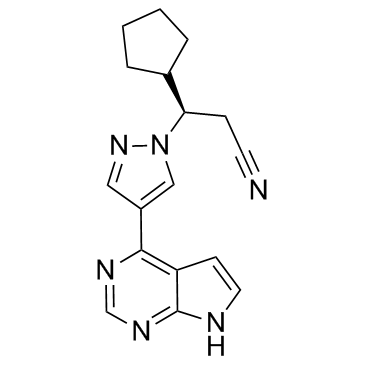
Ruxolitinib (INCB018424) structure
|
Common Name | Ruxolitinib (INCB018424) | ||
|---|---|---|---|---|
| CAS Number | 941678-49-5 | Molecular Weight | 306.365 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 592.6±50.0 °C at 760 mmHg | |
| Molecular Formula | C17H18N6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 312.2±30.1 °C | |
Use of Ruxolitinib (INCB018424)Ruxolitinib is a potent and selective JAK1/2 inhibitor with IC50s of 3.3 nM and 2.8 nM in cell-free assays, and has 130-fold selectivity for JAK1/2 over JAK3. |
| Name | ruxolitinib |
|---|---|
| Synonym | More Synonyms |
| Description | Ruxolitinib is a potent and selective JAK1/2 inhibitor with IC50s of 3.3 nM and 2.8 nM in cell-free assays, and has 130-fold selectivity for JAK1/2 over JAK3. |
|---|---|
| Related Catalog | |
| Target |
JAK2:2.8 nM (IC50) JAK1:3.3 nM (IC50) Tyk2:19 nM (IC50) JAK3:428 nM (IC50) |
| In Vitro | Ruxolitinib potently and selectively inhibits JAK2V617F-mediated signaling and proliferation, markedly increases apoptosis in a dose dependent manner, and at 64 nM results in a doubling of cells with depolarized mitochondria in Ba/F3 cells. Ruxolitinib demonstrates remarkable potency against erythroid colony formation with IC50 of 67 nM, and inhibits proliferating of erythroid progenitors from normal donors and polycythemia vera patients with IC50 values of 407 nM and 223 nM, respectively[1]. |
| In Vivo | Ruxolitinib (180 mg/kg, orally, twice a day) results in survive rate of greater than 90% by day 22 and markedly reduces splenomegaly and circulating levels of inflammatory cytokines, and preferentially eliminated neoplastic cells, resulting in significantly prolonged survival without myelosuppressive or immunosuppressive effects in a JAK2V617F-driven mouse model[1]. In the Ruxolitinib group, the primary end point is reached in 41.9% of patients, as compared with 0.7% in the placebo group in the double-blind trial of myelofibrosis. Ruxolitinib results in maintaining of reduction in spleen volume and improvement of 50% or more in the total symptom score[2]. Ruxolitinib (15 mg twice daily) treatment leads a total of 28% of the patients to have at least a 35% reduction in spleen volume at week 48 in patients with myelofibrosis, as compared with 0% in the group receiving the best available therapy. The mean palpable spleen length has decreased by 56% with Ruxolitinib but has increased by 4% with the best available therapy at week 48. Patients in the ruxolitinib group has an improvement in overall quality-of-life measures and a reduction in symptoms associated with myelofibrosis[3]. |
| Kinase Assay | Recombinant proteins are expressed using Sf21 cells and baculovirus vectors and purified with affinity chromatography. JAK kinase assays use a homogeneous time-resolved fluorescence assay with the peptide substrate (-EQEDEPEGDYFEWLE). Each enzyme reaction is carried out with Ruxolitinib or control, JAK enzyme, 500 nM peptide, adenosine triphosphate (ATP; 1mM), and 2% dimethyl sulfoxide (DMSO) for 1 hour. The 50% inhibitory concentration (IC50) is calculated as INCB018424 concentration required for inhibition of 50% of the fluorescent signal. |
| Cell Assay | Cells are seeded at 2×103/well of white bottom 96-well plates, treated with Ruxolitinib (INCB018424) from DMSO stocks (0.2% final DMSO concentration), and incubated for 48 hours at 37°C with 5% CO2. Viability is measured by cellular ATP determination using the Cell-Titer Glo luciferase reagent or viable cell counting. Values are transformed to percent inhibition relative to vehicle control, and IC50 curves are fitted according to nonlinear regression analysis of the data using PRISM GraphPad. |
| Animal Admin | Mice are fed standard rodent chow and provided with water ad libitum. Ba/F3-JAK2V617F cells (105 per mouse) are inoculated intravenously into 6- to 8-week-old female BALB/c mice. Survival is monitored daily, and moribund mice are humanely killed and considered deceased at time of death. Treatment with vehicle (5% dimethyl acetamide, 0.5% methocellulose) or Ruxolitinib (INCB018424) begin within 24 hours of cell inoculation, twice daily by oral gavage. Hematologic parameters are measured using a Bayer Advia120 analyzed, and statistical significance is determined using Dunnett testing. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 592.6±50.0 °C at 760 mmHg |
| Molecular Formula | C17H18N6 |
| Molecular Weight | 306.365 |
| Flash Point | 312.2±30.1 °C |
| Exact Mass | 306.159302 |
| PSA | 83.18000 |
| LogP | 1.69 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.747 |
| Storage condition | 2-8°C |
| Hazard Codes | Xi |
|---|
|
~65% 
Ruxolitinib (IN... CAS#:941678-49-5 |
| Literature: Zhou, Jiacheng; Liu, Pingli; Lin, Qiyan; Metcalf, Brian W.; Meloni, David; Pan, Yongchun; Xia, Michael; Li, Mei; Yue, Tai-Yuen; Rodgers, James D.; Wang, Haisheng Patent: US2010/190981 A1, 2010 ; Location in patent: Page/Page column 93-94 ; US 20100190981 A1 |
|
~85% 
Ruxolitinib (IN... CAS#:941678-49-5 |
| Literature: Lin, Qiyan; Meloni, David; Pan, Yongchun; Xia, Michael; Rodgers, James; Shepard, Stacey; Li, Mei; Galya, Laurine; Metcalf, Brian; Yue, Tai-N; Liu, Pingli; Zhou, Jiacheng Organic Letters, 2009 , vol. 11, # 9 p. 1999 - 2002 |
|
~98% 
Ruxolitinib (IN... CAS#:941678-49-5 |
| Literature: Zhou, Jiacheng; Liu, Pingli; Lin, Qiyan; Metcalf, Brian W.; Meloni, David; Pan, Yongchun; Xia, Michael; Li, Mei; Yue, Tai-Yuen; Rodgers, James D.; Wang, Haisheng Patent: US2010/190981 A1, 2010 ; Location in patent: Page/Page column 87-88 ; US 20100190981 A1 |
|
~% 
Ruxolitinib (IN... CAS#:941678-49-5 |
| Literature: Organic Letters, , vol. 11, # 9 p. 1999 - 2002 |
|
~% 
Ruxolitinib (IN... CAS#:941678-49-5 |
| Literature: US2010/190981 A1, ; Page/Page column 79 ; US 20100190981 A1 |
|
~% 
Ruxolitinib (IN... CAS#:941678-49-5 |
| Literature: WO2013/23119 A1, ; |
|
~% 
Ruxolitinib (IN... CAS#:941678-49-5 |
| Literature: WO2013/23119 A1, ; |
|
~% 
Ruxolitinib (IN... CAS#:941678-49-5 |
| Literature: WO2013/23119 A1, ; |
| 1H-Pyrazole-1-propanenitrile, β-cyclopentyl-4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-, (βR)- |
| Ruxolitinib |
| ((3R)-3-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]propanenitrile) |
| (3R)-3-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]propionitrile |
| UNII:82S8X8XX8H |
| (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile |
| INCB018424 |
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine structure](https://image.chemsrc.com/caspic/189/3680-69-1.png)
![1H-Pyrazole-1-propanenitrile, β-cyclopentyl-4-[7-[[2-(triMethylsilyl)ethoxy]Methyl]-7H-pyrrolo[2,3-d]pyriMidin-4-yl]-, (βR)- structure](https://image.chemsrc.com/caspic/316/941685-40-1.png)
![(R)-(4-(1-(2-cyano-1-cyclopentylethyl)-1H-pyrazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)methyl pivalate structure](https://image.chemsrc.com/caspic/195/1146629-80-2.png)
![(R)-3-cyclopentyl-3-(4-(7-(hydroxymethyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)propanenitrile structure](https://image.chemsrc.com/caspic/053/1236033-03-6.png)
![3-Cyclopentyl-3-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile structure](https://image.chemsrc.com/caspic/181/941685-39-8.png)
![(betaR)-beta-Cyclopentyl-4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazole-1-propanenitrile (2Z)-2-butenedioate structure](https://image.chemsrc.com/caspic/450/1092939-15-5.png) CAS#:1092939-15-5
CAS#:1092939-15-5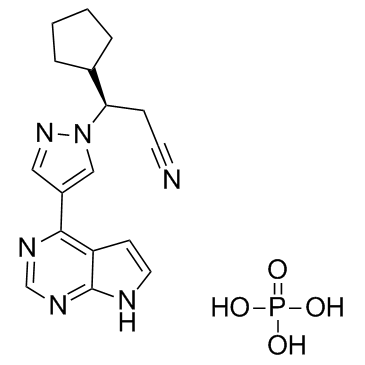 CAS#:1092939-17-7
CAS#:1092939-17-7