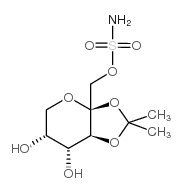
Topiramate

Topiramate structure
|
Common Name | Topiramate | ||
|---|---|---|---|---|
| CAS Number | 97240-79-4 | Molecular Weight | 339.362 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 438.7±55.0 °C at 760 mmHg | |
| Molecular Formula | C12H21NO8S | Melting Point | 125ºC | |
| MSDS | Chinese USA | Flash Point | 219.1±31.5 °C | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of TopiramateTopiramate is an anticonvulsant that antagonizes GluR5 receptors and acts as a positive allosteric modulator of GABA receptor-mediated currents.Target: GluR5 receptor; GABA receptorTopiramate (Topamax) is a structurally novel broad-spectrum antiepileptic drug (AED) with established efficacy as monotherapy or adjunctive therapy in the treatment of adult and paediatric patients with generalised tonic-clonic seizures, partial seizures with or without generalised seizures, and seizures associated with Lennox-Gastaut syndrome [1]. topiramate has been believed to be a type of antiepileptic drug that blocks spread of seizures. Thus far, the mechanisms of its actions have been proven to include use-dependent inhibition of voltage-dependent Na+ channels in neurons, potentiation of GABA (gamma-amino-butyric acid)-induced Cl- influx, and inhibitory effects on inward currents by antagonizing kainate/alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors [2].Topiramate (median average dosage 5.1 mg/kg/day) was also found to be useful as adjunctive therapy in the management of Lennox-Gastaut syndrome and significantly reduced the mean frequency of drop attacks by 14.8% compared with an increase of 5.1% with placebo. Further gains in seizure control were made in a nonblind extension of this trial where the mean topiramate dosage was 10 mg/kg/day. Nine of 11 patients in 1 pilot trial of children with otherwise intractable West syndrome, and 5 of 10 in another, achieved a > or =50% reduction in seizure rate with topiramate (target dosage up to 24 mg/kg/day) [3].Clinical indications: Epilepsy; Lennox Gastaut syndrome; Migraine; Seizure disorder Toxicity:abdominal pain; agitation; blurred vision; convulsions; depression; dizziness; double vision; drowsiness; impaired coordination; impaired mental activity; low blood pressure; reduced consciousness; severe diarrhea; sluggishness and speech problems. |
| Name | topiramate |
|---|---|
| Synonym | More Synonyms |
| Description | Topiramate is an anticonvulsant that antagonizes GluR5 receptors and acts as a positive allosteric modulator of GABA receptor-mediated currents.Target: GluR5 receptor; GABA receptorTopiramate (Topamax) is a structurally novel broad-spectrum antiepileptic drug (AED) with established efficacy as monotherapy or adjunctive therapy in the treatment of adult and paediatric patients with generalised tonic-clonic seizures, partial seizures with or without generalised seizures, and seizures associated with Lennox-Gastaut syndrome [1]. topiramate has been believed to be a type of antiepileptic drug that blocks spread of seizures. Thus far, the mechanisms of its actions have been proven to include use-dependent inhibition of voltage-dependent Na+ channels in neurons, potentiation of GABA (gamma-amino-butyric acid)-induced Cl- influx, and inhibitory effects on inward currents by antagonizing kainate/alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors [2].Topiramate (median average dosage 5.1 mg/kg/day) was also found to be useful as adjunctive therapy in the management of Lennox-Gastaut syndrome and significantly reduced the mean frequency of drop attacks by 14.8% compared with an increase of 5.1% with placebo. Further gains in seizure control were made in a nonblind extension of this trial where the mean topiramate dosage was 10 mg/kg/day. Nine of 11 patients in 1 pilot trial of children with otherwise intractable West syndrome, and 5 of 10 in another, achieved a > or =50% reduction in seizure rate with topiramate (target dosage up to 24 mg/kg/day) [3].Clinical indications: Epilepsy; Lennox Gastaut syndrome; Migraine; Seizure disorder Toxicity:abdominal pain; agitation; blurred vision; convulsions; depression; dizziness; double vision; drowsiness; impaired coordination; impaired mental activity; low blood pressure; reduced consciousness; severe diarrhea; sluggishness and speech problems. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 438.7±55.0 °C at 760 mmHg |
| Melting Point | 125ºC |
| Molecular Formula | C12H21NO8S |
| Molecular Weight | 339.362 |
| Flash Point | 219.1±31.5 °C |
| Exact Mass | 339.098785 |
| PSA | 123.92000 |
| LogP | 2.97 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.497 |
|
SECTION 1: Identification of the substance/mixture and of the company/undertaking Product identifiers Product name: Topiramate REACH No.: A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline.
CAS-No.: 97240-79-4 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Laboratory chemicals, Manufacture of substances SECTION 2: Hazards identification Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 Skin irritation (Category 2), H315 Eye irritation (Category 2), H319 Specific target organ toxicity - single exposure (Category 3), H335 For the full text of the H-Statements mentioned in this Section, see Section 16. Classification according to EU Directives 67/548/EEC or 1999/45/EC Xi IrritantR36/37/38 For the full text of the R-phrases mentioned in this Section, see Section 16. Label elements Labelling according Regulation (EC) No 1272/2008 Pictogram Signal wordWarning Hazard statement(s) H315Causes skin irritation. H319Causes serious eye irritation. H335May cause respiratory irritation. Precautionary statement(s) P261Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray. P305 + P351 + P338IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Supplemental Hazardnone Statements Other hazards - none SECTION 3: Composition/information on ingredients Substances Synonyms: 2,3:4,5-Bis-O-(1-methylethylidene)-36-D-fructo-pyranose sulfamate Topamax McN 4853 RWJ 17021 Formula: C12H21NO8S Molecular Weight: 339,36 g/mol CAS-No.: 97240-79-4 Hazardous ingredients according to Regulation (EC) No 1272/2008 ComponentClassificationConcentration Topiramate CAS-No.97240-79-4Skin Irrit. 2; Eye Irrit. 2; STOT<= 100 % SE 3; H315, H319, H335 Hazardous ingredients according to Directive 1999/45/EC ComponentClassificationConcentration Topiramate CAS-No.97240-79-4Xi, R36/37/38<= 100 % For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16 SECTION 4: First aid measures Description of first aid measures General advice Consult a physician. Show this safety data sheet to the doctor in attendance. If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. In case of skin contact Wash off with soap and plenty of water. Consult a physician. In case of eye contact Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician. If swallowed Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. Most important symptoms and effects, both acute and delayed The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in section 11 Indication of any immediate medical attention and special treatment needed no data available SECTION 5: Firefighting measures Extinguishing media Suitable extinguishing media Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide. Special hazards arising from the substance or mixture Carbon oxides, nitrogen oxides (NOx), Sulphur oxides Advice for firefighters Wear self contained breathing apparatus for fire fighting if necessary. Further information no data available SECTION 6: Accidental release measures Personal precautions, protective equipment and emergency procedures Use personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. For personal protection see section 8. Environmental precautions Do not let product enter drains. Methods and materials for containment and cleaning up Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed containers for disposal. Reference to other sections For disposal see section 13. SECTION 7: Handling and storage Precautions for safe handling Avoid contact with skin and eyes. Avoid formation of dust and aerosols. Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2. Conditions for safe storage, including any incompatibilities Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Recommended storage temperature: 2 - 8 °C Specific end use(s) Apart from the uses mentioned in section 1.2 no other specific uses are stipulated SECTION 8: Exposure controls/personal protection Control parameters Components with workplace control parameters Exposure controls Appropriate engineering controls Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday. Personal protective equipment Eye/face protection Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU). Skin protection Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it. Body Protection impervious clothing, The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Respiratory protection For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU). Control of environmental exposure Do not let product enter drains. SECTION 9: Physical and chemical properties Information on basic physical and chemical properties a) AppearanceForm: solid b) Odourno data available c) Odour Thresholdno data available d) pHno data available e) Melting point/freezingno data available point f) Initial boiling point and no data available boiling range g) Flash pointno data available h) Evapouration rateno data available i) Flammability (solid, gas) no data available j) Upper/lowerno data available flammability or explosive limits k) Vapour pressureno data available l) Vapour densityno data available m) Relative densityno data available n) Water solubilityno data available o) Partition coefficient: n- no data available octanol/water p) Auto-ignitionno data available temperature q) Decompositionno data available temperature r) Viscosityno data available s) Explosive propertiesno data available t) Oxidizing propertiesno data available Other safety information no data available SECTION 10: Stability and reactivity Reactivity no data available Chemical stability Stable under recommended storage conditions. Possibility of hazardous reactions no data available Conditions to avoid no data available Incompatible materials Strong oxidizing agents Hazardous decomposition products Other decomposition products - no data available In the event of fire: see section 5 SECTION 11: Toxicological information Information on toxicological effects Acute toxicity no data available LD50 Intraperitoneal - rat - > 1.500 mg/kg Skin corrosion/irritation no data available Serious eye damage/eye irritation no data available Respiratory or skin sensitisation no data available Germ cell mutagenicity no data available Carcinogenicity IARC:No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC. Reproductive toxicity no data available Specific target organ toxicity - single exposure Inhalation - May cause respiratory irritation. Specific target organ toxicity - repeated exposure no data available Aspiration hazard no data available Additional Information RTECS: LS7083000 To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. SECTION 12: Ecological information Toxicity no data available Persistence and degradability no data available Bioaccumulative potential no data available Mobility in soil no data available Results of PBT and vPvB assessment PBT/vPvB assessment not available as chemical safety assessment not required/not conducted Other adverse effects no data available SECTION 13: Disposal considerations Waste treatment methods Product Offer surplus and non-recyclable solutions to a licensed disposal company. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. Contaminated packaging Dispose of as unused product. SECTION 14: Transport information UN number ADR/RID: -IMDG: -IATA: - UN proper shipping name ADR/RID: Not dangerous goods IMDG: Not dangerous goods IATA:Not dangerous goods Transport hazard class(es) ADR/RID: -IMDG: -IATA: - Packaging group ADR/RID: -IMDG: -IATA: - Environmental hazards ADR/RID: noIMDG Marine pollutant: noIATA: no Special precautions for user no data available SECTION 15 - REGULATORY INFORMATION N/A SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 3 |
| RTECS | LS7083000 |
|
~80% 
Topiramate CAS#:97240-79-4 |
| Literature: EP1627881 A1, ; Page/Page column 3 ; |
|
~76% 
Topiramate CAS#:97240-79-4 |
| Literature: WO2007/99388 A1, ; Page/Page column 6 ; |
|
~% 
Topiramate CAS#:97240-79-4 |
| Literature: WO2004/89965 A2, ; Page title page; 9-10 ; |
|
~61% 
Topiramate CAS#:97240-79-4 |
| Literature: US2006/40874 A1, ; Page/Page column 6-7 ; |
|
~% 
Topiramate CAS#:97240-79-4 |
| Literature: WO2003/97656 A2, ; Page/Page column 101; 102 ; |
|
~29% 
Topiramate CAS#:97240-79-4 |
| Literature: Journal of Medicinal Chemistry, , vol. 41, # 8 p. 1315 - 1343 |
|
Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression.
Nature 457(7231) , 910-4, (2009) Multiple, complex molecular events characterize cancer development and progression. Deciphering the molecular networks that distinguish organ-confined disease from metastatic disease may lead to the i... |
|
|
Kainic Acid-Induced Golgi Complex Fragmentation/Dispersal Shifts the Proteolysis of Reelin in Primary Rat Neuronal Cells: An In Vitro Model of Early Stage Epilepsy.
Mol. Neurobiol. , (2015) The endoplasmic reticulum-lysosome-Golgi network plays an important role in Reelin glycosylation and its proteolytic processing. Golgi complex fragmentation is associated with the separation of Reelin... |
|
|
Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis.
JAMA 311(18) , 1889-900, (2014) Alcohol use disorders cause substantial morbidity and early mortality yet remain greatly undertreated. Medications are considerably underused.To conduct a systematic review and meta-analysis of the be... |
| Topamax Sprinkle |
| 2,3:4,5-Bis-O-(1-methylethylidene)-b-D-fructopyranose sulfamate |
| Topiramate |
| Topimax |
| Tipiramato [Spanish] |
| Epitomax |
| McN-4853 |
| topiramatum [Latin] |
| topiramatum |
| Tipiramate [French] |
| [(3aS,5aR,8aR,8bS)-2,2,7,7-tetramethyl-5,5a,8a,8b-tetrahydrodi[1,3]dioxolo[4,5-a:5',3'-d]pyran-3a-yl]methyl sulfamate |
| Tipiramate |
| [(3aS,5aR,8aR,8bS)-2,2,7,7-Tetramethyltetrahydro-3aH-bis[1,3]dioxolo[4,5-b:4',5'-d]pyran-3a-yl]methyl sulfamate |
| RWJ 17021-000 |
| Topamax |
| Topomax |
| Topiramato [INN-Spanish] |

![N-[(diethylamino)carbonyl]-2,3:4,5-bis-O-(1-methylethylidene)-β-D-fructopyranose sulfamate structure](https://image.chemsrc.com/caspic/255/876403-98-4.png)