柳胺苄心定
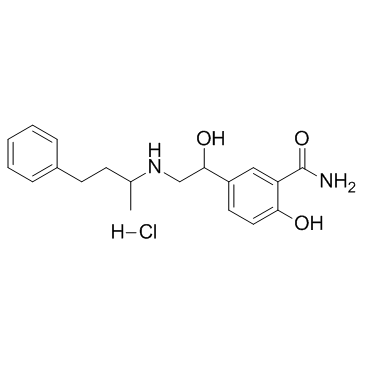
柳胺苄心定结构式

|
常用名 | 柳胺苄心定 | 英文名 | Labetalol HCl |
|---|---|---|---|---|
| CAS号 | 32780-64-6 | 分子量 | 364.87 | |
| 密度 | 1.2±0.1 g/cm3 | 沸点 | 552.7±50.0 °C at 760 mmHg | |
| 分子式 | C19H25ClN2O3 | 熔点 | 187-189° | |
| MSDS | 中文版 美版 | 闪点 | 288.1±30.1 °C |
柳胺苄心定用途Labetalol盐酸盐是一种混合的α/β肾上腺素能拮抗剂,用于治疗高血压。 |
| 中文名 | 盐酸拉贝洛尔 |
|---|---|
| 英文名 | Labetalol hydrochloride |
| 中文别名 | 2-羟基-5-[1-羟基-2-[(1-甲基-3-苯基丙基)氨基]乙基]苯甲酰胺盐酸盐 | 柳胺苄心定 | 柳胺苄心定 |
| 英文别名 | 更多 |
| 描述 | Labetalol盐酸盐是一种混合的α/β肾上腺素能拮抗剂,用于治疗高血压。 |
|---|---|
| 相关类别 |
| 密度 | 1.2±0.1 g/cm3 |
|---|---|
| 沸点 | 552.7±50.0 °C at 760 mmHg |
| 熔点 | 187-189° |
| 分子式 | C19H25ClN2O3 |
| 分子量 | 364.87 |
| 闪点 | 288.1±30.1 °C |
| PSA | 95.58000 |
| LogP | 2.31 |
| 外观性状 | powder | white |
| 蒸汽压 | 0.0±1.6 mmHg at 25°C |
| 折射率 | 1.609 |
| 储存条件 | 2-8°C |
| 水溶解性 | H2O: soluble |
|
模块1. 化学品 1.1 产品标识符 : Labetalol Hydrochloride 产品名称 1.2 鉴别的其他方法 2-Hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]benzamidehydrochloride
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。 模块2. 危险性概述 2.1 GHS-分类 急性毒性, 经口 (类别 5) 2.2 GHS 标记要素,包括预防性的陈述 象形图无 警示词警告 危险申明 H303吞咽可能有害。 警告申明无 2.3 其它危害物 - 无 模块3. 成分/组成信息 3.1 物 质 : 2-Hydroxy-5-[1-hydroxy-2-[(1-methyl-3- 别名 phenylpropyl)amino]ethyl]benzamidehydrochloride : C19H24N2O3 · HCl 分子式 : 364.9 g/mol 分子量 组分浓度或浓度范围 5-[1-Hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]salicylamide monohydrochloride - 化学文摘登记号(CAS 32780-64-6 No.) 251-211-1 EC-编号 模块4. 急救措施 4.1 必要的急救措施描述 一般的建议 请教医生。 向到现场的医生出示此安全技术说明书。 吸入 如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。 皮肤接触 用肥皂和大量的水冲洗。 请教医生。 眼睛接触 用水冲洗眼睛作为预防措施。 食入 切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。 4.2 主要症状和影响,急性和迟发效应 据我们所知,此化学,物理和毒性性质尚未经完整的研究。 4.3 及时的医疗处理和所需的特殊处理的说明和指示 无数据资料 模块5. 消防措施 5.1 灭火介质 灭火方法及灭火剂 用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。 5.2 源于此物质或混合物的特别的危害 无数据资料 5.3 给消防员的建议 如必要的话,戴自给式呼吸器去救火。 5.4 进一步信息 无数据资料 模块6. 泄露应急处理 6.1 作业人员防护措施、防护装备和应急处置程序 使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 避免吸入粉尘。 6.2 环境保护措施 不要让产品进入下水道。 6.3 泄漏化学品的收容、清除方法及所使用的处置材料 收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。 6.4 参考其他部分 丢弃处理请参阅第13节。 模块7. 操作处置与储存 7.1 安全操作的注意事项 避免形成粉尘和气溶胶。 在有粉尘生成的地方,提供合适的排风设备。 7.2 安全储存的条件,包括任何不兼容性 贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。 7.3 特定用途 无数据资料 模块8. 接触控制和个体防护 8.1 容许浓度 最高容许浓度 没有已知的国家规定的暴露极限。 8.2 暴露控制 适当的技术控制 根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。 个体防护设备 眼/面保护 请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。 皮肤保护 戴手套取 手套在使用前必须受检查。 请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品. 使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手 所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。 身体保护 根据危险物质的类型,浓度和量,以及特定的工作场所选择身体保护措施。, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。 呼吸系统防护 不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。 模块9. 理化特性 9.1 基本的理化特性的信息 a) 外观与性状 形状: 固体 b) 气味 无数据资料 c) 气味阈值 无数据资料 d) pH值 无数据资料 e) 熔点/凝固点 无数据资料 f) 沸点、初沸点和沸程 无数据资料 g) 闪点 无数据资料 h) 蒸发速率 无数据资料 i) 易燃性(固体,气体) 无数据资料 j) 高的/低的燃烧性或爆炸性限度 无数据资料 k) 蒸气压 无数据资料 l) 蒸汽密度 无数据资料 m) 密度/相对密度 无数据资料 n) 水溶性 无数据资料 o) n-辛醇/水分配系数 无数据资料 p) 自燃温度 无数据资料 q) 分解温度 无数据资料 r) 粘度 无数据资料 模块10. 稳定性和反应活性 10.1 反应性 无数据资料 10.2 稳定性 无数据资料 10.3 危险反应 无数据资料 10.4 应避免的条件 无数据资料 10.5 不相容的物质 强氧化剂 10.6 危险的分解产物 其它分解产物 - 无数据资料 模块11. 毒理学资料 11.1 毒理学影响的信息 急性毒性 半数致死剂量 (LD50) 经口 - 大鼠 - 2,114 mg/kg 备注: 行为的:睡眠时间改变(包括正位反射的改变)。 行为的:抽搐或对癫痫阈值的影响。 营养与总代谢:变化:体温降低。 皮肤刺激或腐蚀 无数据资料 眼睛刺激或腐蚀 无数据资料 呼吸道或皮肤过敏 无数据资料 生殖细胞致突变性 无数据资料 致癌性 IARC: 此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。 生殖毒性 实验室试验表明有畸胎生成效应 从实验动物的结果看,过度接触能导致生殖紊乱 特异性靶器官系统毒性(一次接触) 无数据资料 特异性靶器官系统毒性(反复接触) 无数据资料 吸入危险 无数据资料 潜在的健康影响 吸入吸入可能有害。 可能引起呼吸道刺激。 摄入如服入是有害的。 皮肤通过皮肤吸收可能有害。 可能引起皮肤刺激。 眼睛可能引起眼睛刺激。 接触后的征兆和症状 据我们所知,此化学,物理和毒性性质尚未经完整的研究。 附加说明 化学物质毒性作用登记: CV5376000 模块12. 生态学资料 12.1 生态毒性 无数据资料 12.2 持久性和降解性 无数据资料 12.3 潜在的生物累积性 无数据资料 12.4 土壤中的迁移性 无数据资料 12.5 PBT 和 vPvB的结果评价 无数据资料 12.6 其它不良影响 无数据资料 模块13. 废弃处置 13.1 废物处理方法 产品 将剩余的和不可回收的溶液交给有许可证的公司处理。 与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧 受污染的容器和包装 按未用产品处置。 模块14. 运输信息 14.1 联合国危险货物编号 欧洲陆运危规: -国际海运危规: -国际空运危规: - 14.2 联合国运输名称 欧洲陆运危规: 非危险货物 国际海运危规: 非危险货物 国际空运危规: 非危险货物 14.3 运输危险类别 欧洲陆运危规: -国际海运危规: -国际空运危规: - 14.4 包裹组 欧洲陆运危规: -国际海运危规: -国际空运危规: - 14.5 环境危险 欧洲陆运危规: 否国际海运危规国际空运危规: 否 海洋污染物(是/否): 否 14.6 对使用者的特别提醒 无数据资料 模块 15 - 法规信息 N/A 模块16 - 其他信息 N/A |
| 个人防护装备 | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| 危害码 (欧洲) | Xn:Harmful |
| 风险声明 (欧洲) | R22 |
| 安全声明 (欧洲) | S36 |
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | 3 |
| RTECS号 | CV5376000 |
| 柳胺苄心定上游产品 0 | |
|---|---|
| 柳胺苄心定下游产品 2 | |
|
Myoglobin microplate assay to evaluate prevention of protein peroxidation.
J. Pharm. Biomed. Anal. 114 , 305-11, (2015) The current therapeutic strategies are based on the design of multifunctional drug candidates able to interact with various disease related targets. Drugs that have the ability to scavenge reactive ox... |
|
|
The alpha- and beta-adrenoceptor blocking activities of labetalol and its RR-SR (50:50) stereoisomers.
Br. J. Pharmacol. 104 , 823-828, (1991) 1. We compared the alpha 1-, alpha 2- and beta 1-adrenoceptor blocking potencies of labetalol with those of its two stereoisomers (RR and SR) in pithed rats and in homogenized rat cerebral cortex and ... |
|
|
Acute hypertension promotes hemorrhagic transformation in a rabbit embolic stroke model: effect of labetalol.
Exp. Neurol. 150 , 153, (1998) We examined the relationship between acute hypertension following cerebral embolization and subsequent hemorrhagic transformation (HT) in a rabbit embolic stroke model. We have shown previously that t... |
| 2-Hydroxy-5-{1-hydroxy-2-[(4-phenylbutan-2-yl)amino]ethyl}benzamide |
| 2-Hydroxy-5-{1-hydroxy-2-[(4-phenylbutan-2-yl)amino]ethyl}benzamide hydrochloride (1:1) |
| Amipress |
| Labetalol hydrochloride |
| benzamide, 2-hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]-, monohydrochloride |
| Labetalol |
| Benzamide, 2-hydroxy-5-(1-hydroxy-2-((1-methyl-3-phenylpropyl)amino)ethyl)- |
| Pressalolo |
| Trandate |
| 2-Hydroxy-5-{1-hydroxy-2-[(4-phenyl-2-butanyl)amino]ethyl}benzamide |
| Salicylamide, 5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]-, hydrochloride |
| Benzamide, 2-hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]-, hydrochloride (1:1) |
| Benzamide, 2-hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]- |
| Labrocol |
| 2-hydroxy-5-{1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl}benzamide hydrochloride |
| 2-Hydroxy-5-{1-hydroxy-2-[(4-phenyl-2-butanyl)amino]ethyl}benzamide hydrochloride (1:1) |
| 2-Hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]benzamide hydrochloride |
| 2-Hydroxy-5-(1-hydroxy-2-((4-phenylbutan-2-yl)amino)ethyl)benzamide hydrochloride |
| 2-hydroxy-5-[1-hydroxy-2-(4-phenylbutan-2-ylamino)ethyl]benzamide,hydrochloride |
| 5-[1-Hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]salicylamide hydrochloride |
| 2-hydroxy-5-{1-hydroxy-2-[(1-méthyl-3-phénylpropyl)amino]éthyl}benzamide chlorhydrate |
| labetolol |
| Ipolab |
| UNII:R5H8897N95 |
| 2-Hydroxy-5-{1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl}benzolcarboxamidhydrochlorid |
| LABETALOL HCL |
| Labetalol (hydrochloride) |


 CAS号22374-89-6
CAS号22374-89-6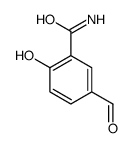 CAS号76143-20-9
CAS号76143-20-9
