盐酸地尔硫卓
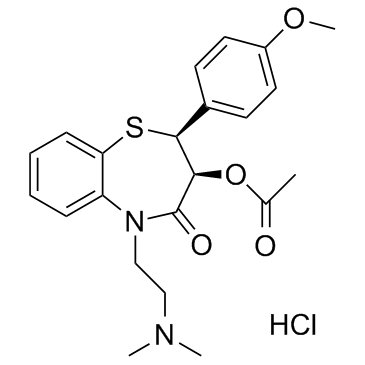
盐酸地尔硫卓结构式

|
常用名 | 盐酸地尔硫卓 | 英文名 | diltiazem hydrochloride |
|---|---|---|---|---|
| CAS号 | 33286-22-5 | 分子量 | 450.979 | |
| 密度 | 1.26g/cm3 | 沸点 | 594.4ºC at 760mmHg | |
| 分子式 | C22H27ClN2O4S | 熔点 | 212-214 °C | |
| MSDS | 中文版 美版 | 闪点 | 313.3ºC | |
| 符号 |


GHS02, GHS07 |
信号词 | Danger |
盐酸地尔硫卓用途Diltiazem hydrochloride是钙离子流入抑制剂(缓慢通道阻断剂或钙拮抗剂)。 |
||||
盐酸地尔硫卓作用1 药理本品为钙离子通道阻滞剂,其作用与心肌及血管平滑肌除极时抑制钙离子内流有关。本品可以有效地扩张心外膜和心内膜下的冠状动脉,缓解自发性心绞痛或由麦角新碱诱发冠状动脉痉挛所致心绞痛;通过减慢心率和降低血压,减少心肌需氧量,增加运动耐量并缓解劳力型心绞痛。本品可以使血管平滑肌松弛,周围血管阻力下降,血压降低;其降压的幅度与高血压的程度有关,血压正常者仅使血压轻度下降。本品有负性肌力作用,并可减慢窦房结和房室结的传导。 2 毒理致癌、致突变和生殖毒性作用有报告大鼠服用本品24个月,小鼠服用本品21个月未发现致癌作用。体外细菌实验未发现致突变作用。动物实验证实本品对生育力无明显作用。 更多
|
| 中文名 | 盐酸地尔硫卓 |
|---|---|
| 英文名 | diltiazem hydrochloride |
| 中文别名 | 顺-(+)-5-[(2-二甲氨基)乙基]-2-(4-甲氧基苯基)-3-乙酰氧基-2,3-二氢-1,5-苯并硫氮杂卓-4(5H)-酮盐酸盐 | (+)-顺式盐酸地尔硫 | 硫氮酮 |
| 英文别名 | 更多 |
| 描述 | Diltiazem hydrochloride是钙离子流入抑制剂(缓慢通道阻断剂或钙拮抗剂)。 |
|---|---|
| 相关类别 | |
| 体外研究 | 苯并硫氮杂卓Ca2 +拮抗剂地尔硫卓盐酸盐与L型Ca2 +通道α1亚基中的跨膜区段IIIS6和IVS6相互作用[1]。地尔硫卓引起剂量依赖性的收缩抑制以及由α肾上腺素能受体激活和高K +去极化刺激的Ca2 +流入。地尔硫卓在抑制高钾和低浓度去甲肾上腺素(NE)引起的收缩方面大致同样有效[2]。地尔硫卓还抑制心脏线粒体中Na依赖性Ca-外流。地尔硫卓的顺式和反式形式的(+)-光学异构体均抑制Na-Ca交换活性,具有相当的效力(IC50为10-20μM)[3]。 |
| 体内研究 | 地尔硫卓对去极化兔主动脉的Ca2 +诱导的收缩产生非竞争性抑制。此外,去除[Ca2 +] ex和添加地尔硫卓的平滑肌作用之间缺乏平行[2]。地尔硫卓可改善大鼠甲亢实验模型中的心脏微循环和功能。用氯沙坦地尔硫卓治疗甲状腺功能亢进大鼠(4.7±0.7%; P <0.001)可显着降低左心室纤维化区域的百分比[4]。在有意识的自发性高血压大鼠(SHR)中,地尔硫卓剂量依赖性地降低血压并且在静脉内施用后增加心率(0.03-1mg/kg)。口服施用地尔硫卓(100 mg/kg)也可降低SHR的血压[5]。 |
| 参考文献 |
| 密度 | 1.26g/cm3 |
|---|---|
| 沸点 | 594.4ºC at 760mmHg |
| 熔点 | 212-214 °C |
| 分子式 | C22H27ClN2O4S |
| 分子量 | 450.979 |
| 闪点 | 313.3ºC |
| 精确质量 | 450.138000 |
| PSA | 84.38000 |
| LogP | 4.23550 |
| 外观性状 | 无气味的白色粉末 |
| 折射率 | 118 ° (C=1, H2O) |
| 储存条件 | 密封、在2 ºC -8 ºC下保存 |
| 稳定性 | 常温常压下稳定 |
| 水溶解性 | soluble |
| 计算化学 | 1、 疏水参数计算参考值(XlogP): 2、 氢键供体数量:1 3、 氢键受体数量:5 4、 可旋转化学键数量:7 5、 互变异构体数量: 6、 拓扑分子极性表面积(TPSA):59.1 7、 重原子数量:30 8、 表面电荷:0 9、 复杂度:565 10、同位素原子数量:0 11、确定原子立构中心数量:2 12、不确定原子立构中心数量:0 13、确定化学键立构中心数量:0 14、不确定化学键立构中心数量:0 15、共价键单元数量:2 |
| 更多 | 1. 性状:无味白色结晶性粉末 2. 密度(g/mL,20℃):未确定 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):212-214 5. 沸点(ºC,常压):未确定 6. 沸点(ºC,KPa):未确定 7. 折射率:118º(C=1,H2O) 8. 闪点(ºC):未确定 9. 比旋光度(º):未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(Pa,20ºC):未确定 12. 饱和蒸气压(KPa,20ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性:易溶于水、甲醇、氯仿,难溶于无水乙醇,不溶于苯。 |
|
模块1. 化学品 1.1 产品标识符 : (+)-cis-Diltiazem hydrochloride 产品名称 1.2 鉴别的其他方法 CRD-401 (2S,3S)-(+)-cis-3-Acetoxy-5-(2-dimethylaminoethyl)-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-
4(5H)-onehydrochloride 1.3 有关的确定了的物质或混合物的用途和建议不适合的用途 仅用于研发。不作为药品、家庭或其它用途。 模块2. 危险性概述 2.1 GHS-分类 急性毒性, 经口 (类别 4) 2.2 GHS 标记要素,包括预防性的陈述 象形图 警示词警告 危险申明 H302吞咽有害。 警告申明 预防 P264操作后彻底清洁皮肤。 P270使用本产品时不要进食、饮水或吸烟。 响应 P301 + P312如果吞下去了: 如感觉不适,呼救解毒中心或看医生。如吞咽:如感觉不适,呼叫解毒中 心或就医。 P330漱口。 处置 P501将内容物/ 容器处理到得到批准的废物处理厂。 2.3 其它危害物 - 无 模块3. 成分/组成信息 3.1 物 质 : CRD-401 别名 (2S,3S)-(+)-cis-3-Acetoxy-5-(2-dimethylaminoethyl)-2,3-dihydro-2-(4- methoxyphenyl)-1,5-benzothiazepin-4(5H)-onehydrochloride : C22H26N2O4S · HCl 分子式 : 450.98 g/mol 分子量 组分浓度或浓度范围 (2S-cis)-3-Acetoxy-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)- one monohydrochloride - 化学文摘登记号(CAS33286-22-5 No.) 251-443-3 EC-编号 模块4. 急救措施 4.1 必要的急救措施描述 一般的建议 请教医生。 向到现场的医生出示此安全技术说明书。 吸入 如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。 皮肤接触 用肥皂和大量的水冲洗。 请教医生。 眼睛接触 用水冲洗眼睛作为预防措施。 食入 切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。 4.2 主要症状和影响,急性和迟发效应 消化系统失调 4.3 及时的医疗处理和所需的特殊处理的说明和指示 无数据资料 模块5. 消防措施 5.1 灭火介质 灭火方法及灭火剂 用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。 5.2 源于此物质或混合物的特别的危害 碳氧化物, 氮氧化物, 硫氧化物, 氯化氢气体 5.3 给消防员的建议 如必要的话,戴自给式呼吸器去救火。 5.4 进一步信息 无数据资料 模块6. 泄露应急处理 6.1 作业人员防护措施、防护装备和应急处置程序 使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 避免吸入粉尘。 6.2 环境保护措施 不要让产品进入下水道。 6.3 泄漏化学品的收容、清除方法及所使用的处置材料 收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。 6.4 参考其他部分 丢弃处理请参阅第13节。 模块7. 操作处置与储存 7.1 安全操作的注意事项 避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。 在有粉尘生成的地方,提供合适的排风设备。 7.2 安全储存的条件,包括任何不兼容性 贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。 建议的贮存温度: 2 - 8 °C 7.3 特定用途 无数据资料 模块8. 接触控制和个体防护 8.1 容许浓度 最高容许浓度 没有已知的国家规定的暴露极限。 8.2 暴露控制 适当的技术控制 根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。 个体防护设备 眼/面保护 带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。 皮肤保护 所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。 戴手套取 手套在使用前必须受检查。 请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品. 使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手 身体保护 全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。 呼吸系统防护 如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国 143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143) 防毒罐。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。 模块9. 理化特性 9.1 基本的理化特性的信息 a) 外观与性状 形状: 粉末 颜色: 白色 b) 气味 无数据资料 c) 气味阈值 无数据资料 d) pH值 无数据资料 e) 熔点/凝固点 熔点/凝固点: 212 °C f) 沸点、初沸点和沸程 无数据资料 g) 闪点 无数据资料 h) 蒸发速率 无数据资料 i) 易燃性(固体,气体) 无数据资料 j) 高的/低的燃烧性或爆炸性限度 无数据资料 k) 蒸气压 无数据资料 l) 蒸汽密度 无数据资料 m) 密度/相对密度 无数据资料 n) 水溶性 大约50 g/l o) n-辛醇/水分配系数 无数据资料 p) 自燃温度 无数据资料 q) 分解温度 无数据资料 r) 粘度 无数据资料 模块10. 稳定性和反应活性 10.1 反应性 无数据资料 10.2 稳定性 无数据资料 10.3 危险反应 无数据资料 10.4 应避免的条件 无数据资料 10.5 不相容的物质 强氧化剂 10.6 危险的分解产物 其它分解产物 - 无数据资料 模块11. 毒理学资料 11.1 毒理学影响的信息 急性毒性 半数致死剂量 (LD50) 经口 - 大鼠 - 560 mg/kg 备注: 行为的:抑制精神的 皮肤刺激或腐蚀 无数据资料 眼睛刺激或腐蚀 无数据资料 呼吸道或皮肤过敏 无数据资料 生殖细胞突变性 无数据资料 致癌性 IARC: 此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。 生殖毒性 无数据资料 特异性靶器官系统毒性(一次接触) 无数据资料 特异性靶器官系统毒性(反复接触) 无数据资料 吸入危险 无数据资料 潜在的健康影响 吸入吸入可能有害。 可能引起呼吸道刺激。 摄入误吞对人体有害。 皮肤通过皮肤吸收可能有害。 可能引起皮肤刺激。 眼睛可能引起眼睛刺激。 接触后的征兆和症状 消化系统失调 附加说明 化学物质毒性作用登记: DL0310000 模块12. 生态学资料 12.1 生态毒性 无数据资料 12.2 持久性和降解性 无数据资料 12.3 潜在的生物累积性 无数据资料 12.4 土壤中的迁移性 无数据资料 12.5 PBT 和 vPvB的结果评价 无数据资料 12.6 其它不良影响 无数据资料 模块13. 废弃处置 13.1 废物处理方法 产品 与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧 将剩余的和不可回收的溶液交给有许可证的公司处理。 受污染的容器和包装 按未用产品处置。 模块14. 运输信息 14.1 联合国危险货物编号 欧洲陆运危规: -国际海运危规: -国际空运危规: - 14.2 联合国运输名称 欧洲陆运危规: 非危险货物 国际海运危规: 非危险货物 国际空运危规: 非危险货物 14.3 运输危险类别 欧洲陆运危规: -国际海运危规: -国际空运危规: - 14.4 包裹组 欧洲陆运危规: -国际海运危规: -国际空运危规: - 14.5 环境危险 欧洲陆运危规: 否国际海运危规国际空运危规: 否 海洋污染物(是/否): 否 14.6 对使用者的特别提醒 无数据资料 模块 15 - 法规信息 N/A 模块16 - 其他信息 N/A |
| 符号 |


GHS02, GHS07 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H225-H302 + H312 + H332-H319 |
| 警示性声明 | P210-P261-P302 + P352 + P312-P304 + P340 + P312-P337 + P313-P403 + P235 |
| 个人防护装备 | dust mask type N95 (US);Eyeshields;Gloves |
| 危害码 (欧洲) | Xn:Harmful |
| 风险声明 (欧洲) | R22;R40 |
| 安全声明 (欧洲) | S36-S45-S36/37 |
| 危险品运输编码 | 3249 |
| WGK德国 | 3 |
| RTECS号 | DL0310000 |
| 包装等级 | III |
| 危险类别 | 6.1(b) |
| 海关编码 | 2934999090 |
1、 由2-氨基硫酚与对甲氧苯基环氧丙酸乙酯缩合得2-(4-甲氧基苯基)-3-羟基-1,5-苯并硫氮杂 -4(5H)-酮。再经酰化、与二甲氨基氯乙烷缩合、成盐制得硫氮卓酮。
| 海关编码 | 2934999090 |
|---|---|
| 中文概述 | 2934999090. 其他杂环化合物. 增值税率:17.0%. 退税率:13.0%. 监管条件:无. 最惠国关税:6.5%. 普通关税:20.0% |
| 申报要素 | 品名, 成分含量, 用途 |
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Characterization of Species Differences in Tissue Diltiazem Deacetylation Identifies Ces2a as a Rat-Specific Diltiazem Deacetylase.
Drug Metab. Dispos. 43 , 1218-25, (2015) Diltiazem, a calcium channel blocker, is mainly metabolized via demethylation or deacetylation in humans. Diltiazem demethylation is catalyzed by cytochrome P450 2D6 and 3A4. Although it was previousl... |
|
|
A predictive ligand-based Bayesian model for human drug-induced liver injury.
Drug Metab. Dispos. 38 , 2302-8, (2010) Drug-induced liver injury (DILI) is one of the most important reasons for drug development failure at both preapproval and postapproval stages. There has been increased interest in developing predicti... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
| Adizem |
| masdil |
| 1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, (2S,3S)-, hydrochloride (1:1) |
| MFCD00069252 |
| Kardil |
| Diltiazem hydrochloride s |
| Dilthiazem hydrochloride |
| (2S,3S)-(+)-cis-3-Acetoxy-5-(2-dimethylaminoethyl)-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one hydrochloride |
| (2S,3S)-5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydro-1,5-benzothiazepin-3-yl acetate hydrochloride |
| (+)-cis-Diltiazem Hydrochloride |
| Cohlen |
| Cormax |
| Zilde |
| Diltiazem HCl |
| angiotrofin |
| EINECS 251-443-3 |
| (2S,3S)-5-[2-(Dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydro-1,5-benzothiazepin-3-ylacetathydrochlorid |
| dilzem |
| bruzem |
| Diltiazem (hydrochloride) |
| (+)-cis-3-(Acetyloxy)-5-(2-(dimethylamino)ethyl)-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one Monohydrochloride |
| Dodexen A.P. |
| 1,5-benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, (2S,3S)-, monohydrochloride |
| Diltiazem d-cis-form hydrochloride |
| Presokin A. P. |
| acétate de (2S,3S)-5-[2-(diméthylamino)éthyl]-2-(4-méthoxyphényl)-4-oxo-2,3,4,5-tétrahydro-1,5-benzothiazépin-3-yle chlorhydrate |
| Tiazac |
| (2S,3S)-5-[2-(Dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydro-1,5-benzothiazepin-3-yl acetate hydrochloride (1:1) |
| Diltiazem hydrochloride |
| Zilden |

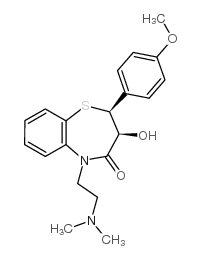 CAS号42399-40-6
CAS号42399-40-6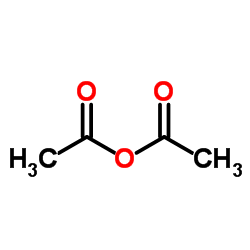 CAS号108-24-7
CAS号108-24-7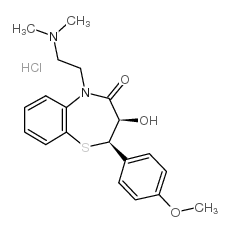 CAS号75472-91-2
CAS号75472-91-2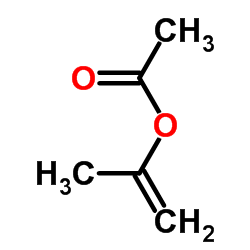 CAS号108-22-5
CAS号108-22-5 CAS号42399-48-4
CAS号42399-48-4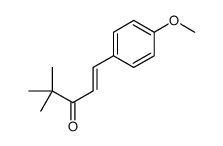 CAS号2419-67-2
CAS号2419-67-2 CAS号123-11-5
CAS号123-11-5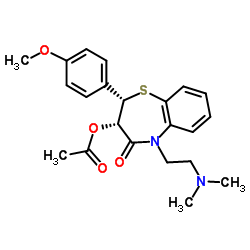 CAS号42399-41-7
CAS号42399-41-7
