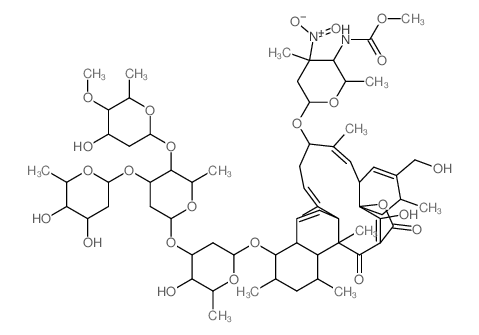
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
OB8650500
-
CHEMICAL NAME :
-
Kijanimicin
-
CAS REGISTRY NUMBER :
-
78798-08-0
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
4
-
MOLECULAR FORMULA :
-
C67-H100-N2-O24
-
MOLECULAR WEIGHT :
-
1317.69
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
125 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - peritonitis
-
REFERENCE :
-
TOXID9 Toxicologist. (Soc. of Toxicology, Inc., 475 Wolf Ledge Parkway, Akron, OH 44311) V.1- 1981- Volume(issue)/page/year: 4,6,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
EPXXDW European Patent Application. (U.S. Patent and Trademark Office, Foreign Patents, Washington, DC 20231) Volume(issue)/page/year: #33840
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
170 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - peritonitis
-
REFERENCE :
-
TOXID9 Toxicologist. (Soc. of Toxicology, Inc., 475 Wolf Ledge Parkway, Akron, OH 44311) V.1- 1981- Volume(issue)/page/year: 4,6,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
EPXXDW European Patent Application. (U.S. Patent and Trademark Office, Foreign Patents, Washington, DC 20231) Volume(issue)/page/year: #33840
|