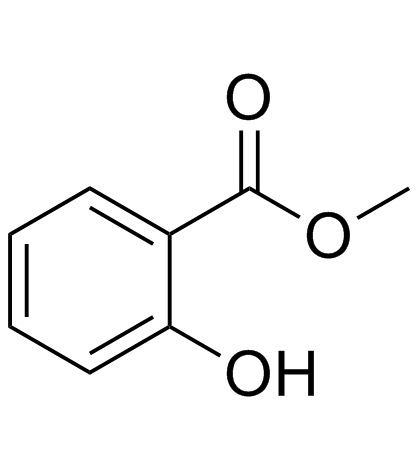
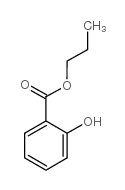
毒理学数据:
对小儿的平均致死量为10mL,成人为30mL。豚鼠经口LD50为0.7g/kg。兔经口LD50为2.8g/kg。嗅觉阈浓度0.0037mg/m3。
大鼠经口LD50:887mg/kg
生态学数据:
对皮肤有刺激作用。蒸气或烟雾对眼睛、黏膜和上呼吸道有刺激作用。慢性中毒时引起恶心、呕吐、酸中毒症、呼吸器官疾病、昏睡以致死亡。
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
VO4725000
-
CHEMICAL NAME :
-
Salicylic acid, methyl ester
-
CAS REGISTRY NUMBER :
-
119-36-8
-
BEILSTEIN REFERENCE NO. :
-
0971516
-
LAST UPDATED :
-
199710
-
DATA ITEMS CITED :
-
45
-
MOLECULAR FORMULA :
-
C8-H8-O3
-
MOLECULAR WEIGHT :
-
152.16
-
WISWESSER LINE NOTATION :
-
QR BVO1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
Standard Draize test
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
TYPE OF TEST :
-
Standard Draize test
-
ROUTE OF EXPOSURE :
-
Administration into the eye
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
TYPE OF TEST :
-
Standard Draize test
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
TYPE OF TEST :
-
Standard Draize test
-
ROUTE OF EXPOSURE :
-
Administration into the eye
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
101 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Gastrointestinal - nausea or vomiting
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
1329 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Behavioral - coma Blood - hemorrhage
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
228 mg/kg
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - dyspnea Gastrointestinal - nausea or vomiting
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
700 mg/kg
-
TOXIC EFFECTS :
-
Peripheral Nerve and Sensation - flaccid paralysis without anesthesia (usually neuromuscular blockage) Behavioral - general anesthetic Lungs, Thorax, or Respiration - dyspnea
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
355 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - coma Lungs, Thorax, or Respiration - respiratory stimulation Gastrointestinal - nausea or vomiting
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - infant
-
DOSE/DURATION :
-
1480 mg/kg
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - respiratory stimulation Gastrointestinal - nausea or vomiting
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
506 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
522 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
887 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity)
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1110 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
2100 mg/kg
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - other changes Gastrointestinal - hypermotility, diarrhea Gastrointestinal - nausea or vomiting
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
2250 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
1300 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
4250 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
700 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
1500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
71400 mg/kg/17W-C
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
546 gm/kg/2Y-C
-
TOXIC EFFECTS :
-
Cardiac - changes in heart weight Kidney, Ureter, Bladder - changes in kidney weight Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TCLo - Lowest published toxic concentration
-
ROUTE OF EXPOSURE :
-
Inhalation
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
40 mg/m3/4H/17W-I
-
TOXIC EFFECTS :
-
Blood - changes in erythrocyte (RBC) count Blood - changes in leukocyte (WBC) count Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - dehydrogenases
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
85200 mg/kg/10W-C
-
TOXIC EFFECTS :
-
Musculoskeletal - other changes Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
40800 mg/kg/59D-I
-
TOXIC EFFECTS :
-
Liver - fatty liver degeneration Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
330 gm/kg/90D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - changes in structure or function of endocrine pancreas Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
36450 mg/kg
-
SEX/DURATION :
-
multigenerations
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
400 mg/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
500 mg/kg
-
SEX/DURATION :
-
female 11-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
500 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - body wall Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
500 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - hepatobiliary system Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1750 mg/kg
-
SEX/DURATION :
-
female 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
5250 mg/kg
-
SEX/DURATION :
-
female 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System
MUTATION DATA
-
TYPE OF TEST :
-
Mutation in microorganisms
-
TEST SYSTEM :
-
Bacteria - Salmonella typhimurium
-
DOSE/DURATION :
-
100 ug/disc
-
REFERENCE :
-
JNUDAT Journal of Nihon University School of Dentistry. (Nihon University School of Dentistry, 1-8-13 Surugadai, Kanda, Chiyoda-ku, Tokyo, 101, Japan) V.1- 1958- Volume(issue)/page/year: 34,183,1992 *** REVIEWS *** TOXICOLOGY REVIEW DICPBB Drug Intelligence and Clinical Pharmacy. (POB 42435, Cincinnati, OH 45242) V.3- 1969- Volume(issue)/page/year: 9,350,1975 TOXICOLOGY REVIEW CLCHAU Clinical Chemistry (Winston-Salem, NC). (American Assoc. for Clinical Chemistry, 1725 K St., NW, Washington, DC 20006) V.1- 1955- Volume(issue)/page/year: 19,361,1973 TOXICOLOGY REVIEW ARVPAX Annual Review of Pharmacology. (Palo Alto, CA) V.1-15, 1961-75. For publisher information, see ARPTDI. Volume(issue)/page/year: 5,447,1965 TOXICOLOGY REVIEW AJMEAZ American Journal of Medicine. (Technical Pub., 875 Third Ave., New York, NY 10022) V.1- 1946- Volume(issue)/page/year: 38,409,1965 TOXICOLOGY REVIEW EVHPAZ EHP, Environmental Health Perspectives. (U.S. Government Printing Office, Supt of Documents, Washington, DC 20402) No.1- 1972- Volume(issue)/page/year: 15,121,1976 TOXICOLOGY REVIEW FAATDF Fundamental and Applied Toxicology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1981- Volume(issue)/page/year: 13,747,1989 *** U.S. STANDARDS AND REGULATIONS *** EPA FIFRA 1988 PESTICIDE SUBJECT TO REGISTRATION OR RE-REGISTRATION FEREAC Federal Register. (U.S. Government Printing Office, Supt. of Documents, Washington, DC 20402) V.1- 1936- Volume(issue)/page/year: 54,7740,1989 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 48320 No. of Facilities: 15668 (estimated) No. of Industries: 138 No. of Occupations: 80 No. of Employees: 105490 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 48320 No. of Facilities: 30724 (estimated) No. of Industries: 193 No. of Occupations: 134 No. of Employees: 454822 (estimated) No. of Female Employees: 134722 (estimated)
|