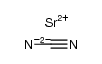
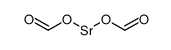
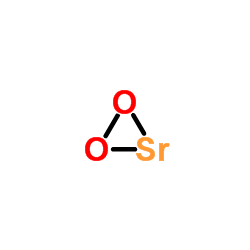
1633-05-2
| 中文名 | 碳酸锶 |
|---|---|
| 英文名 | Strontium carbonate |
| 中文别名 | 菱锶矿 |
| 英文别名 |
strontium,carbonate
MFCD00011250 Carbonic acid, strontium salt (1:1) Strontium carbonate EINECS 216-643-7 |
| 密度 | 3.7 g/mL at 25 °C(lit.) |
|---|---|
| 沸点 | 333.6ºC at 760 mmHg |
| 熔点 | 1497°C |
| 分子式 | CO3Sr |
| 分子量 | 147.629 |
| 闪点 | 169.8ºC |
| 精确质量 | 147.890366 |
| PSA | 63.19000 |
| 外观性状 | 白色或灰色粉末 |
| 蒸汽压 | 2.58E-05mmHg at 25°C |
| 储存条件 | 保持容器密封,储存在阴凉,干燥的地方 内衬塑料薄膜的编袋装包装,每袋净重25kg、50kg、100kg,密封存贮于阴凉干燥处,防止包装破裂。 |
| 稳定性 | 1.菱锶矿是一种碳酸盐矿物。 菱锶矿化学组成SrO 70.19%、CO2 29.81%,常含锰、钡和钙。硬度35,性脆,相对密度3.76。通常为白色,有时因含杂质,而成灰、黄、白、浅绿或褐色等,条痕白色。斜方晶系完全菱面体解理,玻璃光泽,断面呈油脂光泽,贝壳状断口。溶于稀盐酸,产生气泡。与方铅矿、闪锌矿和黄铜矿伴生于含硫化物的矿脉中,也与方解石、白云石以及石英伴生。 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:0 3.氢键受体数量:3 4.可旋转化学键数量:0 5.互变异构体数量:无 6.拓扑分子极性表面积63.2 7.重原子数量:5 8.表面电荷:0 9.复杂度:18.8 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:2 |
| 更多 | 1. 性状:无色斜方晶系,或白色细微粉末。无臭。无味 2. 密度(g/mL,25/4℃): 3.7 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):1700 5. 沸点(ºC,常压):2647 6. 沸点(ºC,5.2kPa): 未确定 7. 折射率: 未确定 8. 闪点(ºC): 未确定 9. 比旋光度(º): 未确定 10. 自燃点或引燃温度(ºC): 未确定 11. 蒸气压(kPa,25ºC): 未确定 12. 饱和蒸气压(kPa,60ºC): 未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC): 未确定 15. 临界压力(KPa): 未确定 16. 油水(辛醇/水)分配系数的对数值: 未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V): 未确定 19. 溶解性:难溶于水,微溶于氨水、碳酸铵和CO2 饱和水溶液,不溶于醇, |
Synonym:None known Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: 34 Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Causes burns.Corrosive. Potential Health Effects Eye: Causes eye burns. May cause chemical conjunctivitis and corneal damage. Skin: Causes skin burns. May cause skin rash (in milder cases), and cold and clammy skin with cyanosis or pale color. Ingestion: May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract burns. May cause perforation of the digestive tract. May cause systemic effects. Inhalation: May cause severe irritation of the respiratory tract with sore throat, coughing, shortness of breath and delayed lung edema. Causes chemical burns to the respiratory tract. Aspiration may lead to pulmonary edema. May cause systemic effects. Chronic: Effects may be delayed. Section 4 - FIRST AID MEASURES Eyes: Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed. Extensive irrigation with water is required (at least 30 minutes). Skin: Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes. Ingestion: Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately. Inhalation: Get medical aid immediately. Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask. Notes to Physician: Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use extinguishing media appropriate to the surrounding fire. Substance is noncombustible. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Extinguishing Media: Substance is noncombustible; use agent most appropriate to extinguish surrounding fire. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation. Section 7 - HANDLING and STORAGE Handling: Use only in a well-ventilated area. Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Keep container tightly closed. Do not ingest or inhale. Discard contaminated shoes. Storage: Keep container closed when not in use. Store in a cool, dry, well-ventilated area away from incompatible substances. Corrosives area. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 1633-05-2: Russia: 6 mg/m3 TWA CAS# 7647-01-0: United Kingdom, WEL - TWA: 1 ppm TWA (aerosol mist and gas); 2 mg TWA (aerosol mist and gas) United Kingdom, WEL - STEL: 5 ppm STEL (aerosol mist and gas); 8 mg/m3 STEL (aerosol mist and gas) United States OSHA: ; 5 ppm Ceiling; 7 mg/m3 Ceiling Belgium - TWA: 5 ppm VLE; 8 mg/m3 VLE Belgium - STEL: 10 ppm VLE; 15 mg/m3 VLE France - VLE: 5 ppm VLE; 7.5 mg/m3 VLE Germany: 8 mg/m3 TWA Japan: 5 ppm Ceiling; 7.5 mg/m3 Ceiling Malaysia: 5 ppm Ceiling; 7.5 mg/m3 Ceiling Netherlands: 10 ppm STEL; 15 mg/m3 STEL Netherlands: 5 ppm MAC; 8 mg/m3 MAC Russia: 5 mg/m3 TWA Spain: 5 ppm VLA-ED; 7.6 mg/m3 VLA-ED Spain: 10 ppm VLA-EC; 15 mg/m3 VLA-EC CAS# 7732-18-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure. Respirators: A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Liquid Color: colorless Odor: None reported. pH: <1.0 Vapor Pressure: Not available. Viscosity: Not available. Boiling Point: Not available. Freezing/Melting Point: Not available. Autoignition Temperature: Not applicable. Flash Point: Not applicable. Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: Solubility in water: miscible Specific Gravity/Density: Molecular Formula: Solution Molecular Weight: 0 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable under normal temperatures and pressures. Conditions to Avoid: Incompatible materials, excess heat. Incompatibilities with Other Materials: Strong acids, strong bases, strong oxidizing agents. Hazardous Decomposition Products: Hydrogen chloride. Hazardous Polymerization: Has not been reported. Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 1633-05-2: WK8305000 CAS# 7647-01-0: MW4025000 MW4031000 CAS# 7732-18-5: ZC0110000 LD50/LC50: Not available. CAS# 7647-01-0: Inhalation, mouse: LC50 = 1108 ppm/1H; Inhalation, mouse: LC50 = 20487 mg/m3/5M; Inhalation, mouse: LC50 = 3940 mg/m3/30M; Inhalation, mouse: LC50 = 8300 mg/m3/30M; Inhalation, rat: LC50 = 3124 ppm/1H; Inhalation, rat: LC50 = 60938 mg/m3/5M; Inhalation, rat: LC50 = 7004 mg/m3/30M; Inhalation, rat: LC50 = 45000 mg/m3/5M; Inhalation, rat: LC50 = 8300 mg/m3/30M; Oral, rabbit: LD50 = 900 mg/kg. CAS# 7732-18-5: Oral, rat: LD50 = >90 mL/kg. Carcinogenicity: Strontium carbonate - Not listed by ACGIH, IARC, or NTP. Hydrogen chloride - Not listed by ACGIH, IARC, or NTP. Water - Not listed by ACGIH, IARC, or NTP. Other: See actual entry in RTECS for complete information. Section 12 - ECOLOGICAL INFORMATION Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. Section 14 - TRANSPORT INFORMATION IATA Shipping Name: HYDROCHLORIC ACID Hazard Class: 8 UN Number: 1789 Packing Group: II IMO Shipping Name: HYDROCHLORIC ACID Hazard Class: 8 UN Number: 1789 Packing Group: II RID/ADR Shipping Name: HYDROCHLORIC ACID Hazard Class: 8 UN Number: 1789 Packing group: II USA RQ: CAS# 7647-01-0: 5000 lb final RQ; 2270 kg final RQ Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: C Risk Phrases: R 34 Causes burns. Safety Phrases: S 24/25 Avoid contact with skin and eyes. WGK (Water Danger/Protection) CAS# 1633-05-2: 0 CAS# 7647-01-0: 1 CAS# 7732-18-5: No information available. Canada CAS# 1633-05-2 is listed on Canada's DSL List. CAS# 7647-01-0 is listed on Canada's DSL List. CAS# 7732-18-5 is listed on Canada's DSL List. CAS# 1633-05-2 is not listed on Canada's Ingredient Disclosure List. CAS# 7647-01-0 is listed on Canada's Ingredient Disclosure List. CAS# 7732-18-5 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 1633-05-2 is listed on the TSCA inventory. CAS# 7647-01-0 is listed on the TSCA inventory. CAS# 7732-18-5 is listed on the TSCA inventory. SECTION 16 - ADDITIONAL INFORMATION N/A |
|
毒理学数据: 吸入锶化合物粉尘,能引起两肺中度弥漫性间质改变。最高容许浓度为6mg/m3。 生态学数据: 通常对水是不危害的,若无政府许可,勿将材料排入周围环境。
|
| 个人防护装备 | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | - |
| 海关编码 | 2836920000 |
|
~% 
1633-05-2 |
| 文献:Zeitschrift fuer Physikalische Chemie, Abteilung A: Chemische Thermodynamik, Kinetik, Elektrochemie, Eigenschaftslehre, , vol. 183, p. 153 - 161 |
|
~% 
1633-05-2 |
| 文献:Zeitschrift fuer Elektrochemie, , vol. 38, p. 240 - 247 Z. Anorg. Chem., , vol. 215, p. 415 - 426 |
|
~% 
1633-05-2 |
| 文献:Journal of Physics and Chemistry of Solids, , vol. 47, p. 409 - 412 |
|
~% 
1633-05-2
详细
|
| 文献:Materials Research Bulletin, , vol. 37, # 15 p. 2517 - 2524 |
|
~% 
1633-05-2 |
| 文献:Techn. Kurir (ungar.), , vol. 9, p. 71 - 74 |
|
~% 
1633-05-2 |
| 文献:Zeitschrift fuer Elektrochemie, , vol. 38, p. 240 - 247 |
|
~0% 
1633-05-2
详细
|
| 文献:Thermochimica Acta, , vol. 284, # 1 SPEC. ISS. p. 213 - 227 |
|
~% 
1633-05-2
详细
|
| 文献:Journal of Thermal Analysis, , vol. 32, p. 1243 - 1252 |
| 上游产品 7 | |
|---|---|
| 下游产品 7 | |
| 海关编码 | 2836920000 |
|---|