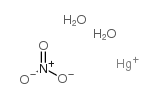
14836-60-3
| 中文名 | 二水硝酸汞 |
|---|---|
| 英文名 | mercury(1+),nitric acid,dihydrate |
| 中文别名 |
二水硝酸亚汞(I)
硝酸亚汞二水合物 |
| 英文别名 |
EINECS 233-886-4
Nitric acid,mercury(1+) salt,dihydrate MFCD00150812 nitric acid,mercury salt,hydrate (1:1:2) |
| 密度 | 4,79 g/cm3 |
|---|---|
| 沸点 | 83ºC at 760mmHg |
| 熔点 | 70 °C |
| 分子式 | H4HgNO5 |
| 分子量 | 298.62500 |
| 精确质量 | 299.98000 |
| PSA | 87.34000 |
| LogP | 0.15300 |
| 外观性状 | 潮湿的灰白色晶体,温和气味 |
| 蒸汽压 | 49.8mmHg at 25°C |
| 储存条件 | 常温密闭避光,通风干燥 |
| 稳定性 | 1.常温常压下稳定 避免的物料:水分/潮湿 还原剂 易氧化材料 有机材料 酸 硫磺 光 2.溶于水和稀硝酸,但在大量水中则分解为碱式盐的沉淀。氨和氢氧化钠溶液能使之变黑。有毒,吸入或与皮肤接触时有极毒,并有蓄积性危害。 |
| 分子结构 | 1、摩尔折射率:无可用的 2、摩尔体积(cm3/mol):无可用的 3、等张比容(90.2K):无可用的 4、表面张力(dyne/cm):无可用的 5、介电常数:无可用的 6、极化率(10-24cm3):无可用的 7、单一同位素质量:299.979547 Da 8、标称质量:300 Da 9、平均质量:298.6255 Da |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:3 3.氢键受体数量:5 4.可旋转化学键数量:0 5.互变异构体数量:无 6.拓扑分子极性表面积68 7.重原子数量:7 8.表面电荷:1 9.复杂度:24.8 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:4 |
| 更多 | 1.性状:未确定 2.密度(g/mL,25/4℃):4.79 3.相对蒸汽密度(g/mL,空气=1):未确定 4.熔点(ºC):70 5.沸点(ºC,常压):未确定 6.沸点(ºC,5.2kPa):未确定 7.折射率:未确定 8.闪点(ºC):未确定 9.比旋光度(º):未确定 10.自燃点或引燃温度(ºC):未确定 11.蒸气压(kPa,25ºC):未确定 12.饱和蒸气压(kPa,60ºC):未确定 13.燃烧热(KJ/mol):未确定 14.临界温度(ºC):未确定 15.临界压力(KPa):未确定 16.油水(辛醇/水)分配系数的对数值:未确定 17.爆炸上限(%,V/V):未确定 18.爆炸下限(%,V/V):未确定 19.溶解性:溶于水和稀硝酸,不溶于乙醇和乙醚。 |
|
Section 1. Chemical Product and Company Identification Mercurous Nitrate, Dihydrate Common Name/ Trade Name Mercurous Nitrate, Dihydrate Section 3. Hazards Identification Very hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion. Hazardous in case of inhalation.
Potential Acute Health Slightly hazardous in case of skin contact (permeator). Prolonged exposure may result in skin burns and ulcerations. Effects Over-exposure by inhalation may cause respiratory irritation. Severe over-exposure can result in death. Inflammation of the eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or, occasionally, blistering. CARCINOGENIC EFFECTS: Not available. Potential Chronic Health MUTAGENIC EFFECTS: Not available. Effects TERATOGENIC EFFECTS: Not available. DEVELOPMENTAL TOXICITY: Not available. The substance may be toxic to kidneys, the nervous system, gastrointestinal tract, skin, central nervous system (CNS). Repeated or prolonged exposure to the substance can produce target organs damage. Repeated exposure to a highly toxic material may produce general deterioration of health by an accumulation in one or many human organs. Section 4. First Aid Measures Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least Eye Contact 15 minutes. Get medical attention immediately. In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated Skin Contact clothing and shoes. Cover the irritated skin with an emollient. Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention immediately. Wash with a disinfectant soap and cover the contaminated skin with an anti-bacterial cream. Seek immediate Serious Skin Contact medical attention. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Serious InhalationEvacuate the victim to a safe area as soon as possible. Loosen tight clothing such as a collar, tie, belt or waistband. If breathing is difficult, administer oxygen. If the victim is not breathing, perform mouth-to-mouth resuscitation. Seek medical attention. If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to Ingestion an unconscious person. Loosen tight clothing such as a collar, tie, belt or waistband. Get medical attention immediately. Not available. Serious Ingestion Section 5. Fire and Explosion Data Flammability of the Product Non-flammable. Auto-Ignition Temperature Not applicable. Not applicable. Flash Points Not applicable. Flammable Limits Not available. Products of Combustion Fire Hazards in Presence of of combustible materials of organic materials Various Substances Risks of explosion of the product in presence of mechanical impact: Not available. Explosion Hazards in Risks of explosion of the product in presence of static discharge: Not available. Presence of Various Substances Not applicable. Fire Fighting Media and Instructions Contact with combustible or organic materials may cause fire. When heated to decomposition it emits toxic fumes of Special Remarks on nitrogen oxides, mercury/mercury oxides Fire Hazards Contact with red hot carbon causes mild explosion. Special Remarks on Mixture of Mercurous nitrate and Phosphorus explodes violently when struck with hammer. Explosion Hazards Mercurous Nitrate, Dihydrate Section 6. Accidental Release Measures Use appropriate tools to put the spilled solid in a convenient waste disposal container. Small Spill Large SpillOxidizing material. Poisonous solid. Stop leak if without risk. Do not get water inside container. Avoid contact with a combustible material (wood, paper, oil, clothing...). Keep substance damp using water spray. Do not touch spilled material. Use water spray to reduce careful that the product is not present at a concentration level above TLV. Check TLV on the MSDS and with local authorities. Section 7. Handling and Storage Keep away from heat. Keep away from sources of ignition. Keep away from combustible material.. Do not ingest. Precautions Do not breathe dust. In case of insufficient ventilation, wear suitable respiratory equipment. If ingested, seek medical advice immediately and show the container or the label. Avoid contact with skin and eyes. Keep away from incompatibles such as reducing agents, combustible materials, organic materials, metals. Keep container tightly closed. Keep container in a cool, well-ventilated area. Separate from acids, alkalies, Storage reducing agents and combustibles. See NFPA 43A, Code for the Storage of Liquid and Solid Oxidizers. Sensitive to light. Store in light-resistant containers. Section 8. Exposure Controls/Personal Protection Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below Engineering Controls recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSplash goggles. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used to avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE of a Large Spill handling this product. Exposure Limits for Inorganic Mercury Compounds (as Hg): Exposure Limits TWA: 0.025 (mg(Hg)/m3) from ACGIH (TLV) [United States] SKIN (skin designation). Skin absorption as potential significant contribution to overall exposure. TWA: 0.1 (mg(Hg)/m3) from ACGIH (TLV) [United States] Inhalation TWA: 0.1 (mg(Hg)/m3) from NIOSH [United States] SKIN (skin designation). Skin absorption as potential significant contribution to overall exposure. TWA: 0.1 (mg(Hg)/m3) from OSHA (PEL) [United States] Inhalation TWA: 0.05 STEL: 0.15 (mg(Hg)/m3) [United Kingdom (UK)] Consult local authorities for acceptable exposure limits. Section 9. Physical and Chemical Properties Solid. (Crystals solid.)Not available. Physical state andO dor appearance Not available. Taste 561.22 g/mole Molecular Weight Not available. Color Not available. pH (1% soln/water) Not available. Boiling Point Decomposition temperature: 70°C (158°F) Melting Point Not available. Critical Temperature 4.78 (Water = 1) Specific Gravity Not applicable. Vapor Pressure Not available. Vapor Density Not available. Volatility Not available. Odor Threshold Mercurous Nitrate, Dihydrate Not available. Water/Oil Dist. Coeff. Not available. Ionicity (in Water) Not available. Dispersion Properties Soluble in 13 parts water containing 1% Nitric acid.. Solubility Insoluble in ammonium hydroxide. Section 10. Stability and Reactivity Data The product is stable. Stability Not available. Instability Temperature Conditions of Instability Incompatible materials Incompatibility with various Reactive with reducing agents, combustible materials, organic materials, metals, acids. substances Non-corrosive in presence of glass. Corrosivity Effloresces and becomes anhydrous in dry air. Special Remarks on Incompatible with phosphorus, ammonia, most common metals, strong reducing agents, combustible materials, Reactivity organic materials, cyanide, thiocyanates, isothiocyanates, hypophosphites, hyphosphoric acid Not available. Special Remarks on Corrosivity Will not occur. Polymerization Section 11. Toxicological Information Absorbed through skin. Inhalation. Ingestion. Routes of Entry Toxicity to AnimalsAcute oral toxicity (LD50): 49.3 mg/kg [Mouse]. Acute dermal toxicity (LD50): 2330 mg/kg [Rat]. Chronic Effects on Humans May cause damage to the following organs: kidneys, the nervous system, gastrointestinal tract, skin, central nervous system (CNS). Very hazardous in case of skin contact (irritant), of ingestion. Other Toxic Effects on HumansHazardous in case of inhalation. Slightly hazardous in case of skin contact (permeator). Not available. Special Remarks on Toxicity to Animals May cause adverse reproductive effects and birth defects (teratogenic) Special Remarks on Chronic Effects on Humans Acute Potential Health Effects: Special Remarks on other Skin: Causes irritation and possible burns. It can be absorbed through the skin with symptoms similar to ingestion. Toxic Effects on Humans Harmful if absorbed through skin. Eyes: Causes irritation with possible burns and eye damage. Inhalation: Can cause respiratory tract (nose, throat, lung) irritation causing sore throat, coughing, tightness in chest, breathing difficulities, and/or shortness of breath. Pneumonitis may develop. Ingestion: Toxic. Harmful if swallowed. May cause burining of the mouth and pharynx. Can cause salivation, metallic taste, abdominal pain, nausea, vomiting, hypermotility, bloody diarrhea. May affect the kidneys (proteinuria, acute renal failure). Inhalation/Ingestion: High or repeated exposure can cause Mercury poisoning. Mercury poisoning causes sore gums, personality changes, tremor/"shakes" (often with shaky handwriting), clumsiness, fatigue, irritability and increased saliva. Other changes may include serious personality changes memory loss, extreme shyness, weakness, stomatitis, gingivitis, loss of teeth, gastrointestinal disturbances, metallic taste, poor appetite/anorexia, weight loss, "pins and needles" (peripheral neuropathy). Exposures can also affect the liver, cause kidney damage, and may cause decreased visual acuity, and affect peripheral vision (the ability to see to the side). Brain damage can occur, especially if exposure continues. Chronic Potential Health Effects Mercurous Nitrate, Dihydrate Inhalation/Ingestion: High or repeated exposure can cause Mercury poisoning. See above for symtoms of Mercury poisoning. Eye Contact: brown staining in the eye without visual impairment. Skin: Repeated skin contact can make the skin turn gray. Skin allergy/dermatitis can also occur. If this happens, even small future exposures can cause rash. Note: In addition to the effects of exposure to mercury, this product is also a nitrate. The first clinical signs associated with nitrate poisoning include: Gastroenteritis, abdominal pain, nausea, vomiting(spontaneous vomiting), diarrhea, metabolic acidosis. Purging and diuresis can be expected. The toxicity of nitrates is due to the in vivo conversion to nitrites. The primary toxic effects of nitrites include orthostatic hypotension (due to perpheral vasodilation) and methemoglobinemia (the formation of methemoglobin in the blood which causes deficient oxygenation of the blood due to decreased available hemoglobin). Other symptoms may include muscular weakness, dizziness, lightheadness, fatigue, throbbing headache, mental impairment, incoordination, seizures convulsions, bradycardia or tachydardia (slow or fast heart beat), dysrhythmias, dyspnea. Furthermore, methemoglobinemia due to inadequate oxygenation of the blood can lead to progressive cyanosis, and coma. Cyanosis is first visible as a bluish discoloration of the mucous membranes and unpigmented areas of the body. Prolonged or repeated ingestion of large amounts of nitrates may affect the liver and can cause nausea, vomiting, anorexia/weight loss, methemoglobinemia (characterized by dizziness, rapid or slow heart beat, irregular breathing, convulsions), and possible coma and death. Section 12. Ecological Information Not available. Ecotoxicity Not available. BOD5 and COD Products of Biodegradation Possibly hazardous short term degradation products are not likely. However, long term degradation products may arise. The products of degradation are less toxic than the product itself. Toxicity of the Products of Biodegradation Not available. Special Remarks on the Products of Biodegradation Section 13. Disposal Considerations Waste must be disposed of in accordance with federal, state and local environmental control Waste Disposal regulations. Section 14. Transport Information CLASS 6.1: Poisonous material. DO T Cl assi fi cati on : Mercurous nitrate UNNA: 1627 PG: II Identification Marine Pollutant Special Provisions for Transport DO T (Pi ctograms) Section 15. Other Regulatory Information and Pictograms California prop. 65: This product contains the following ingredients for which the State of California has found to Federal and State cause cancer, birth defects or other reproductive harm, which would require a warning under the statute: Mercurous Regulations Nitrate, Dihydrate (Listed as Mercury and Mercury compounds) California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: Mercurous Nitrate, Dihydrate (Listed as Mercury and Mercury compounds) Connecticut hazardous material survey.: Mercurous Nitrate (CAS number 10415-75-5) Illinois chemical safety act: Mercurous Nitrate (CAS number 10415-75-5) New York release reporting list: Mercurous Nitrate (CAS number 10415-75-5) Pennsylvania RTK: Mercurous Nitrate (CAS number 10415-75-5) Massachusetts RTK: Mercurous Nitrate (CAS number 10415-75-5) Mercurous Nitrate, Dihydrate Massachusetts spill list: Mercurous Nitrate (CAS number 10415-75-5) New Jersey: Mercurous Nitrate (CAS number 10415-75-5) New Jersey spill list: Mercurous Nitrate (CAS number 10415-75-5) Louisiana spill reporting: Mercurous Nitrate (CAS number 10415-75-5) California Director's List of Hazardous Substances: Mercurous Nitrate (CAS number 10415-75-5) SARA 313 toxic chemical notification and release reporting: Mercurous Nitrate, Dihydrate (Listed as Mercury and Mercury compounds) CERCLA: Hazardous substances.: Mercurous Nitrate: 10 lbs. (4.536 kg) California prop. 65: This product contains the following ingredients for which the State of California has found to California cause cancer which would require a warning under the statute: No products were found. Proposition 65 Warnings California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: Mercurous Nitrate, Dihydrate (Listed as Mercury and Mercury compounds) OSHA: Hazardous by definition of Hazard Communication Standard (29 CFR 1910.1200). Other Regulations The classification of this product has not been validated yet by the Service du repertoire Other ClassificationsWHMIS (Canada) toxicologique. However, it might be classified as: CLASS C: Oxidizing material. CLASS D-1A: Material causing immediate and serious toxic effects (VERY TOXIC). CLASS D-2B: Material causing other toxic effects (TOXIC). R8- Contact with combustible materialS26- In case of contact with eyes, rinse DSCL (EEC) may cause fire.immediately with plenty of water and seek R20/21- Harmful by inhalation and inmedical advice. contact with skin.S36/37/39- Wear suitable protective clothing, R25- Toxic if swallowed.gloves and eye/face protection. R33- Danger of cumulative effects.S45- In case of accident or if you feel unwell, R36/38- Irritating to eyes and skin.seek medical advice immediately (show the R50/53- Very toxic to aquaticlabel where possible). organisms, may cause long-termS60- This material and its container must be adverse effects in the aquaticdisposed of as hazardous waste. environment.S61- Avoid release to the environment. Refer to special instructions/Safety data sheets. HMIS (U.S.A.)Health Hazard3 National Fire Protection 0 Flammability 0 Association (U.S.A.) Fire Hazard 3 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) TDG(Canada) (Pictograms) ADR (Europe) (Pictograms) Mercurous Nitrate, Dihydrate Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Wear appropriate respirator when ventilation is inadequate. SECTION 16 - ADDITIONAL INFORMATION N/A |
|
生态学数据: 对水体是极其危害的,即使小量产品不能接触地下水,水道或污水系统,未经政府许可勿将材料排入周围环境。
|
| 危害码 (欧洲) | N: Dangerous for the environment;T+: Very toxic; |
|---|---|
| 风险声明 (欧洲) | R50/53 |
| 安全声明 (欧洲) | S61-S60-S45-S13 |
| 危险品运输编码 | 1627 |
| 包装等级 | II |
| 危险类别 | 6.1 |