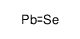12069-00-0
| 中文名 | 硒化铅(II) |
|---|---|
| 英文名 | lead selenide |
| 中文别名 | 硒化铅 |
| 英文别名 |
MFCD00016273
EINECS 235-109-4 |
| 密度 | 8.1 g/cm3 |
|---|---|
| 熔点 | 1065ºC |
| 分子式 | HPbSe |
| 分子量 | 287.16800 |
| 精确质量 | 288.90100 |
| 外观性状 | 灰色粉末和片,无气味 |
| 储存条件 | 常温密闭,阴凉通风干燥 |
| 稳定性 | 常温常压下稳定 避免光,明火,高温。不溶于水,微溶于硝酸,具高毒性,怕湿气,具半导体性质。 |
| 分子结构 | 1、摩尔折射率:无可用的 2、摩尔体积(cm3/mol):无可用的 3、等张比容(90.2K):无可用的 4、表面张力(dyne/cm):无可用的 5、介电常数:无可用的 6、极化率(10-24cm3):无可用的 7、单一同位素质量:287.893122 Da 8、标称质量:288 Da 9、平均质量:286.16 Da |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:0 3.氢键受体数量:0 4.可旋转化学键数量:0 5.互变异构体数量:无 6.拓扑分子极性表面积0 7.重原子数量:2 8.表面电荷:0 9.复杂度:2 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1. 性状:铅灰色立方晶系晶体。 2. 密度(g/mL,25℃):8.10 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):1078 5. 沸点(ºC,常压):未确定 6. 沸点(ºC,1mmHg):未确定 7. 折射率:未确定 8. 闪点(ºC):未确定 9. 比旋光度(º):未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(20ºC):未确定 12. 饱和蒸气压(kPa,60ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性:溶于硝酸和热浓盐酸中,不溶于水。 |
|
Section 1: Product Identification Chemical Name:Lead (II) selenide (99.99%-Pb)PURATREM CAS Registry Number:12069-00-0 Formula:PbSe EINECS Number:235-109-4 Chemical Family:metal chalcogenides Synonym:none
Section 2: Composition and Information on Ingredients IngredientCAS NumberPercentACGIH (TWA)OSHA (PEL) Title Compound12069-00-0100%0.05mg/m3 (as Pb)0.05mg/m3 (as Pb) Section 3: Hazards Identification Harmful by inhalation and if swallowed. Chronic effects are severe and include headache, anemia, brain Emergency Overview: damage and reproductive disorders. May cause cancer. Danger of cumulative effects. Primary Routes of Exposure:Ingestion, skin, inhalation of dust. Eye Contact:May cause slight to mild irritation of the eyes. Skin Contact:May cause slight to mild irritation of the skin Inhalation:Large dust exposure may cause encephalopathy, then seizures, coma, and cardiorespiratory distress. Ingestion may lead to dizziness, abdominal cramps, vomiting, bloody diarrhea, metallic taste, weakness, and Ingestion: convulsions. Exposure may cause abdominal pain, loss of appetite, muscle weakness, headache, joint pain, metallic taste, Acute Health Affects: and encephalopathy (swelling of the brain). May cause harm to the unborn child. The chronic effects of lead poisoning include diarrhea, loss of appetite, muscle pain, headache, dizziness, Chronic Health Affects: anemia, brain damage, and reproductive disorders. Possible risk of impaired fertility. May cause cancer. NTP:No IARC:No OSHA:No SECTION 4: First Aid Measures Immediately flush the eyes with copious amounts of water for at least 10-15 minutes. A victim may need Eye Exposure: assistance in keeping their eye lids open. Get immediate medical attention. Wash the affected area with water. Remove contaminated clothes if necessary. Seek medical assistance if Skin Exposure: irritation persists. Remove the victim to fresh air. Closely monitor the victim for signs of respiratory problems, such as difficulty Inhalation: in breathing, coughing, wheezing, or pain. In such cases seek immediate medical assistance. Seek medical attention immediately. Keep the victim calm. Give the victim water (only if conscious). Induce Ingestion: vomiting only if directed by medical personnel. SECTION 5: Fire Fighting Measures Flash Point:not applicable Autoignition Temperature:no data Explosion Limits:no data Extinguishing Medium:carbon dioxide or dry powder. Fire fighters should be equipped with a NIOSH approved positive pressure self-contained breathing apparatus Special Fire Fighting Procedures: and full protective clothing. Hazardous Combustion andIf involved in a fire this material may emit toxic lead oxide dust. Decomposion Products: Unusual Fire or Explosion Hazards: No unusual fire or explosion hazards. SECTION 6: Accidental Release Measures Small spills can be mixed with vermiculite, sodium carbonate or other suitable non combustible adsorbent and Spill and Leak Procedures: swept up. SECTION 7: Handling and Storage Handling and Storage:No special precautions needed in handling and storage. SECTION 8: Exposure Controls and Personal Protection Eye Protection:Always wear approved safety glasses when handling a chemical substance in the laboratory. Skin Protection:Wear protective clothing and gloves. Consult with glove manufacturer to determine the proper type of glove. Ventilation:Handle the material in an efficient fume hood. If ventilation is not available a respirator should be worn. The use of respirators requires a Respirator Respirator: Protection Program to be in compliance with 29 CFR 1910.134. Ventilation:Handle the material in an efficient fume hood. Additional Protection:No additional protection required. SECTION 9: Physical and Chemical Properties Color and Form:gray to black pieces Molecular Weight:286.15 Melting Point:1065° Boiling Point:no data Vapor Pressure:not applicable Specific Gravity:8.1 Odor:none Solubility in Water:insoluble SECTION 10: Stability and Reactivity Stability:air and moisture stable Hazardous Polymerization:no hazardous polymerization Conditions to Avoid:none Incompatibility:oxidizing agents Decomposition Products:lead oxide and selenium oxide fumes SECTION 11: Toxicological Information RTECS Data:No information available in the RTECS files. Carcinogenic Effects:Carcinogen (as Pb) Mutagenic Effects:No data available Tetratogenic Effects:Reproductive effector (as Pb) SECTION 12: Ecological Information Avoid release to the environment. Very toxic to aquatic organisms. May cause long-term adverse effects in the Ecological Information: aquatic environment. SECTION 13: Disposal Considerations Disposal:Dispose of according to federal, state, and local regulations. SECTION 14: Transportation Shipping Name (CFR):Selenium compound, N.O.S. Hazard Class (CFR):6.1 Additional Hazard Class (CFR):NA Packaging Group (CFR):III UN ID Number (CFR):UN# 3283 Shipping Name (IATA):Selenium compound, Solid, N.O.S. Hazard Class (IATA):6.1 Additional Hazard Class (IATA):NA Packaging Group (IATA):III UN ID Number (IATA):UN# 3283 SECTION 15: Regulatory Information TSCA:Listed in the TSCA inventory. SARA (Title 313):See Category Codes N420 and N725 for reporting. Second Ingredient:none SECTION 16 - ADDITIONAL INFORMATION N/A |
|
毒理学数据: 主要的刺激性影响: 在皮肤上面:刺激皮肤和粘膜,造成腐蚀性影响 在眼睛上面:刺激的影响,造成腐蚀性影响 致敏作用:没有已知的敏化现象 生态学数据: 通常对水体是极其有害的,即使小量产品也不能接触地下水,水道或污水系统
|
| 符号 |



GHS06, GHS08, GHS09 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H301 + H331-H302 + H332-H360Df-H373-H410 |
| 警示性声明 | Missing Phrase - N15.00950417-P201-P260-P280-P304 + P340 + P312-P308 + P313 |
| 个人防护装备 | dust mask type N95 (US);Eyeshields;Gloves |
| 危害码 (欧洲) | Xn |
| 风险声明 (欧洲) | 20/22-33 |
| 安全声明 (欧洲) | 22-26-28-36/37/39-45 |
| 危险品运输编码 | UN 3288 |
| 包装等级 | II |
| 危险类别 | 6.1(a) |