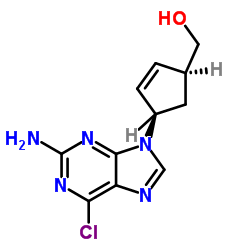
阿巴卡韦杂质A
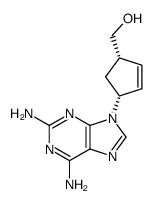
阿巴卡韦杂质A结构式

|
常用名 | 阿巴卡韦杂质A | 英文名 | 6-aminocarbovir |
|---|---|---|---|---|
| CAS号 | 124752-25-6 | 分子量 | 246.26800 | |
| 密度 | N/A | 沸点 | N/A | |
| 分子式 | C11H14N6O | 熔点 | 180-182°C | |
| MSDS | N/A | 闪点 | N/A | |
| 符号 |

GHS05 |
信号词 | Danger |
| 中文名 | 阿巴卡韦杂质A |
|---|---|
| 英文名 | (1α,4α)-4-(2,6-Diamino-9H-purin-9-yl)-2-cyclopentenyl-carbinol |
| 英文别名 | 更多 |
| 熔点 | 180-182°C |
|---|---|
| 分子式 | C11H14N6O |
| 分子量 | 246.26800 |
| 精确质量 | 246.12300 |
| PSA | 115.87000 |
| LogP | 1.26260 |
| 蒸汽压 | 1.21E-16mmHg at 25°C |
| 储存条件 | -20?C Freezer |
| 符号 |

GHS05 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H318 |
| 警示性声明 | P280-P305 + P351 + P338 |
| 危害码 (欧洲) | Xi |
| 风险声明 (欧洲) | 41-43 |
| 安全声明 (欧洲) | 26-36/37-39 |
| 危险品运输编码 | NONH for all modes of transport |
|
~97% 
阿巴卡韦杂质A 124752-25-6 |
| 文献:EMORY UNIVERSITY Patent: WO2009/21114 A1, 2009 ; Location in patent: Page/Page column 44; 47 ; |
|
~98% 
阿巴卡韦杂质A 124752-25-6 |
| 文献:Ando, Takayuki; Iwata, Masafumi; Zulfiqar, Fazila; Miyamoto, Tatsuya; Nakanishi, Masayuki; Kitade, Yukio Bioorganic and Medicinal Chemistry, 2008 , vol. 16, # 7 p. 3809 - 3815 |
|
~74% 
阿巴卡韦杂质A 124752-25-6 |
| 文献:Vince; Brownell; Beers Nucleosides and Nucleotides, 1995 , vol. 14, # 1-2 p. 39 - 44 |
|
~% 
阿巴卡韦杂质A 124752-25-6 |
| 文献:Nucleosides and Nucleotides, , vol. 14, # 1-2 p. 39 - 44 |
| 阿巴卡韦杂质A上游产品 3 | |
|---|---|
| 阿巴卡韦杂质A下游产品 0 | |
|
Comparative brain exposure to (-)-carbovir after (-)-carbovir or (-)-6-aminocarbovir intravenous infusion in rats.
Pharm. Res. 12(6) , 911-5, (1995) Evaluate the ability of (-)-6-aminocarbovir ((-)-6AC) to improve the CNS exposure to (-)-carbovir ((-)-CBV).Activation of (-)-6AC in vitro was assessed by incubations of rat brain tissue homogenates. ... |
|
|
Pharmacokinetic evaluation of (-)-6-aminocarbovir as a prodrug for (-)-carbovir in rats.
Drug Metab. Dispos. 20(1) , 47-51, (1992) The recently synthesized carbocyclic 2',3'-didehydro-2',3'-dideoxy-6-deoxy-6-amino-guanosine [(-)6AC] was evaluated as a prodrug for carbovir, carbocyclic 2',3'-didehydro-2',3'-dideoxyguanosine [(-)CB... |
|
|
First-pass disposition of (-)-6-aminocarbovir in rats. I. Prodrug activation may be limited by access to enzyme.
Drug Metab. Dispos. 27(1) , 113-21, (1999) Several in vitro and in situ approaches were used to determine the dominant presystemic activation site for (-)-6-aminocarbovir, (-)-carbocyclic 2',3'-didehydro-2', 3'-dideoxy-6-deoxy-6-aminoguanosine... |
| ABACAVIR RELATED COMPOUND |
| Descyclopropyl Abacavir |
| Abacavir Impurity 3 |
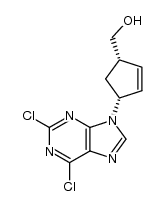
![9-[cis-(1'R,4'S)-4'-acetoxymethyl-2'-cyclopenten-1'-yl]-9H-2-amino-6-chloropurine结构式](https://image.chemsrc.com/caspic/108/162992-44-1.png)