Orphenadrine-d3 hydrochloride
Modify Date: 2024-04-09 19:19:15
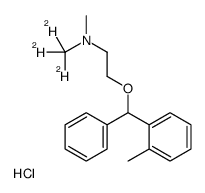
Orphenadrine-d3 hydrochloride structure
|
Common Name | Orphenadrine-d3 hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 1309283-23-5 | Molecular Weight | 308.86100 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C18H21ClD3NO | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Orphenadrine-d3 hydrochlorideOrphenadrine-d3 (hydrochloride) is the deuterium labeled Orphenadrine hydrochloride[1]. Orphenadrine hydrochloride is an orally active and non-competitive NMDA receptor antagonist (crosses the blood-brain barrier) with a Ki of 6.0 μM. Orphenadrine hydrochloride relieves stiffness, pain and discomfort due to muscle strains, sprains or other injuries. Orphenadrine hydrochloride is also used to relieve tremors associated with parkinson's disease. Orphenadrine citrate has good neuroprotective properties, can be used in studies of neurodegenerative diseases[2][3]. |
| Name | N-Methyl-N-(2H3)methyl-2-[(2-methylphenyl)(phenyl)methoxy]ethanamine hydrochloride (1:1) |
|---|
| Description | Orphenadrine-d3 (hydrochloride) is the deuterium labeled Orphenadrine hydrochloride[1]. Orphenadrine hydrochloride is an orally active and non-competitive NMDA receptor antagonist (crosses the blood-brain barrier) with a Ki of 6.0 μM. Orphenadrine hydrochloride relieves stiffness, pain and discomfort due to muscle strains, sprains or other injuries. Orphenadrine hydrochloride is also used to relieve tremors associated with parkinson's disease. Orphenadrine citrate has good neuroprotective properties, can be used in studies of neurodegenerative diseases[2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Molecular Formula | C18H21ClD3NO |
|---|---|
| Molecular Weight | 308.86100 |
| Exact Mass | 308.17300 |
| PSA | 12.47000 |
| LogP | 4.46460 |