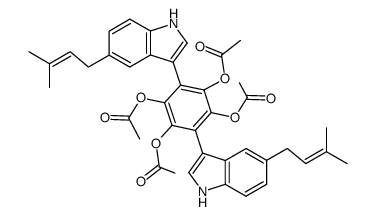
11051-88-0
| Name | 2,5-dihydroxy-3,6-bis[5-(3-methylbut-2-enyl)-1H-indol-3-yl]cyclohexa-2,5-diene-1,4-dione |
|---|---|
| Synonyms |
2,5-dihydroxy-3,6-bis-[5-(3-methyl-but-2-enyl)-indol-3-yl]-[1,4]benzoquinone
Cochliodinol 3,6-Bis(5-(3-methyl-2-butenyl)indol-3-yl)-2,5-dihydroxy-p-benzoquinone p-Benzoquinone,3,6-bis(5-(3-methyl-2-butenyl)indol-3-yl)-2,5-dihydroxy chochliodinol 2,5-Cyclohexadiene-1,4-dione,2,5-dihydroxy-3,6-bis(5-(3-methyl-2-butenyl)-1H-indol-3-yl) 2,5-Dihydroxy-3,6-bis(5-(3-methyl-2-butenyl)-1H-indol-3-yl)-2,5-cyclohexadiene-1,4-dione |
| Description | Cochliodinol (compound 1) is a metabolite derived from the Apis mellifera ligustica. Cochliodinol has strong free radical scavenging activity of 2, 2-diphenyl-1-picrohydrazine (DPPH) (IC50=3.06 μg/mL)[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.344g/cm3 |
|---|---|
| Boiling Point | 790ºC at 760mmHg |
| Molecular Formula | C32H30N2O4 |
| Molecular Weight | 506.59 |
| Flash Point | 431.6ºC |
| Exact Mass | 506.22100 |
| PSA | 106.18000 |
| LogP | 7.05680 |
| Vapour Pressure | 2.66E-26mmHg at 25°C |
| Index of Refraction | 1.73 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 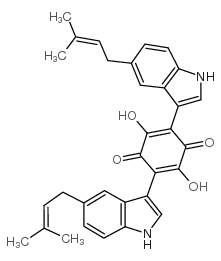
11051-88-0 |
| Literature: Horcher; Schwenner; Franck 1986 , vol. 1986, # 10 p. 1765 - 1771 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |