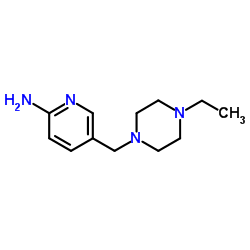
1231929-97-7
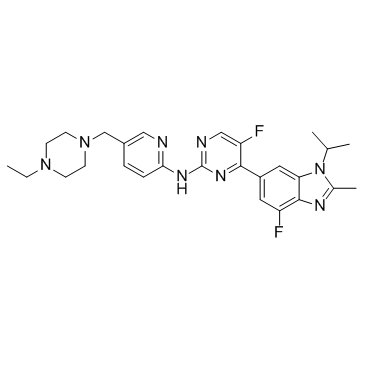
| Name | N-[5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl]-5-fluoro-4-(7-fluoro-2-methyl-3-propan-2-ylbenzimidazol-5-yl)pyrimidin-2-amine |
|---|---|
| Synonyms |
Abemaciclib
2-Pyrimidinamine, N-[5-[(4-ethyl-1-piperazinyl)methyl]-2-pyridinyl]-5-fluoro-4-[4-fluoro-2-methyl-1-(1-methylethyl)-1H-benzimidazol-6-yl]- UNII-60UAB198HK 2-Pyrimidinamine,N-[5-[(4-ethyl-1-piperazinyl)methyl]-2-pyridinyl]-5-fluoro-4-[4-fluoro-2-methyl-1-(1-methylethyl)-1H-benzimidazol-6-yl] N-{5-[(4-Ethyl-1-piperazinyl)methyl]-2-pyridinyl}-5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzimidazol-6-yl)-2-pyrimidinamine LY2835219 [5-(4-ethyl-piperazin-1-ylmethyl)-pyridin-2-yl]-[5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-3H-benzoimidazol-5-yl)-pyrimidin-2-yl]-amine CS-1230 N-[5-[(4-Ethyl-1-piperazinyl)methyl]-2-pyridinyl]-5-fluoro-4-[4-fluoro-2-methyl-1-(1-methylethyl)-1H-benzimidazol-6-yl]-2-pyrimidinamine |
| Description | Abemaciclib (LY2835219) is a selective CDK4/6 inhibitor with IC50 values of 2 nM and 10 nM for CDK4 and CDK6, respectively. |
|---|---|
| Related Catalog | |
| Target |
Cdk4/cyclin D1:2 nM (IC50) CDK6/cyclinD1:10 nM (IC50) CDK9/cyclinT1:57 nM (IC50) CDK5/p35:287 nM (IC50) Cdk5/p25:355 nM (IC50) CDK2/cyclinE:504 nM (IC50) CDK7/Mat1/cyclinH1:3910 nM (IC50) CDK1/cyclinB1:1627 nM (IC50) PIM1:50 nM (IC50) PIM2:3400 nM (IC50) HIPK2:31 nM (IC50) DYRK2:61 nM (IC50) CK2:117 nM (IC50) GSK3b:192 nM (IC50) JNK3:389 nM (IC50) FLT3 (D835Y):403 nM (IC50) FLT3:3960 nM (IC50) DRAK1:659 nM (IC50) |
| In Vitro | Abemaciclib reduces cell viability with the IC50 values ranging from 0.5 μM to 0.7 μM, inhibits Akt and ERK signaling but not mTOR activation at head and neck squamous cell carcinoma (HNSCC) cells[1]. Abemaciclib shows inhibition on A375R1-4, M14R, and SH4R with EC50 values ranging from 0.3 to 0.6 μM; Abemaciclib inhibits the proliferation of the parental A375 and resistant A375RV1 and A375RV2 cells with similar potencies with IC50 values of 395, 260, and 463 nM, respectively[2]. Abemaciclib inhibits CDK4 and CDK6 with low nanomolar potency, inhibits Rb phosphorylation resulting in a G1 arrest and inhibition of proliferation, and its activity is specific for Rb-proficient cells[3]. |
| In Vivo | Abemaciclib (45 mg/kg, p.o.) in combination with everolimus causes a cooperative antitumor effect in HNSCC xenograft tumor[1]. Abemaciclib (45 or 90 mg/kg, p.o.) shows significant tumor growth inhibition in an A375 xenograft model[2]. |
| Cell Assay | Cells are seeded in a 96-well plate, allowed to adhere overnight, and treated with DMSO control (0.1% v/v) or the indicated compounds for 72 h. Cell viability and proliferation are determined using a Cell Counting Kit according to the manufacturer's instructions. The interaction between Abemaciclib and mTOR inhibitor is determined using CompuSyn. Combination index (CI) values of 1 indicates and additive drug interaction, whereas a CI of <1 is synergistic and a CI of >1 is antagonistic. |
| Animal Admin | Six-week-old BALB/c female nude mice are injected subcutaneously with OSC-19 (1×106) cells. When tumor sizes reach approximately 100 mm3, mice are randomized by tumor size and subjected to each treatment. At least 5 mice per treatment group are included. Each group of mice is dosed via daily oral gavage with vehicle, Abemaciclib (45 mg/kg/d or 90 mg/kg/d), Everolimus (5 mg/kg/d), or a combination of both. The Abemaciclib is dissolved in 1% HEC in 20 mM phosphate buffer (pH2.0). Tumor size and body weight are measured twice weekly. Tumor volumes are calculated using the following formula: V=(L×W2)/2. Mice are gavaged a final time on day 14 and sacrificed the following day. The tumors are removed for Western blot and immunohistochemistry. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 689.3±65.0 °C at 760 mmHg |
| Molecular Formula | C27H32F2N8 |
| Molecular Weight | 506.593 |
| Flash Point | 370.7±34.3 °C |
| Exact Mass | 506.271790 |
| PSA | 75.00000 |
| LogP | 2.74 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.656 |
| Hazard Codes | Xi |
|---|
|
~% 
1231929-97-7 |
| Literature: COATES, David A.; GELBERT, Lawrence Mark; KNOBELOCH, John M.; DE DIOS MAGANA, Alfonso; DE PRADO GONZALEZ, Ana; FILADELFA DEL PRADO CATALINA, Miriam; GARCIA PAREDES, Maria Cristina; MARTIN DE LA NAVA, Eva Maria; MARTIN ORTEGA FINGER, Maria Dolores; MARTINEZ PEREZ, Jose Antonio; MATEO HERRANZ, Ana Isabel; PEREZ MARTINEZ, Carlos; SANCHEZ MARTINEZ, Concepcion Patent: US2010/160340 A1, 2010 ; Location in patent: Page/Page column 14 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |