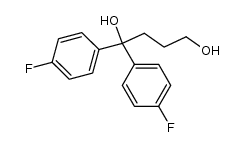
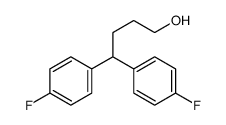
1841-19-6
| Name | Fluspirilene |
|---|---|
| Synonyms |
8-[4,4-Bis(4-fluorophenyl)butyl]-1-phenyl-1,3,8-triazaspiro[4.5]decan-4-one
fluspirilene 8-[4,4-bis(p-Fluorophenyl)butyl]-1-phenyl-1,3,8-triazino[4.5]decan-4-one,R 6218 8-[4,4-bis(p-Fluorophenyl)butyl]-1-phenyl-1,3,8-triazino[4.5]decan-4-one R 6218 1,3,8-Triazaspiro[4.5]decan-4-one, 8-[4,4-bis(4-fluorophenyl)butyl]-1-phenyl- 1,3,8-Triazaspiro(4.5)decan-4-one, 8-(4,4-bis(4-fluorophenyl)butyl)-1-phenyl- |
| Description | Fluspirilene is a non-competitive antagonist of L-type calcium channels with an IC50 of 0.03 μM. Fluspirileneis a long-acting injectable depot antipsychotic drug used for schizophrenia. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.03 μM (L-type calcium channel)[1] |
| In Vitro | Fluspirilene, at concentrations which non-competitively modify dihydropyridine binding, selectively antagonizes the effects of calcium-channel activators[1]. Fluspirilene decreases the viability and suppresses sphere-forming of glioma stem cell lines in a dose-dependent manner. Fluspirilene demonstrates the inhibition of proliferation of T98, U87 and all GSC lines at 1.25, 2.5, and 5 μM, while it inhibits the proliferation of U251 and SNB19 at 2.5 and 5 μM[2]. |
| In Vivo | Mice treated with fluspirilene shows a remarkable reduction of the tumor size. Fluspirilene significantly prolongs survival of the TGS04 mouse model[2]. |
| Cell Assay | To investigate the effect of fluspirilene on cell proliferation, cells are treated with 1.25, 2.5, and 5 μM of fluspirilene. GSC viability is assessed using a Cell Counting Kit-8[2]. |
| Animal Admin | Mice[2] The mice are randomly assigned to two groups and treated with either fluspirilene (n=5) or with DMSO as a control group (n=5). All mice are given intramuscular injections of 200 μL of DMSO or fluspirilene dissolved in DMSO at 1 mg/kg body weight four times[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 668.9±55.0 °C at 760 mmHg |
| Melting Point | 187.5-190° |
| Molecular Formula | C29H31F2N3O |
| Molecular Weight | 475.573 |
| Flash Point | 358.3±31.5 °C |
| Exact Mass | 475.243530 |
| PSA | 35.58000 |
| LogP | 3.89 |
| Appearance | amorphous solid | white to yellow |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.632 |
| Storage condition | room temp |
| Water Solubility | DMSO: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | UN 3249 |
| WGK Germany | 3 |
| RTECS | XX8750000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
|
~97% 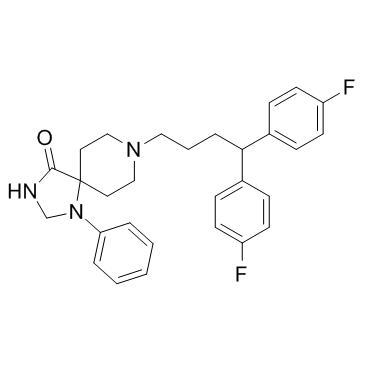
1841-19-6 |
| Literature: Schmidt, Andreas; Marchetti, Mauro; Eilbracht, Peter Tetrahedron, 2004 , vol. 60, # 50 p. 11487 - 11492 |
|
~68% 
1841-19-6 |
| Literature: Botteghi, Carlo; Marchetti, Mauro; Paganelli, Stefano; Persi-Paoli, Francesco Tetrahedron, 2001 , vol. 57, # 8 p. 1631 - 1637 |
|
~% 
1841-19-6 |
| Literature: Tetrahedron, , vol. 60, # 50 p. 11487 - 11492 |
|
~% 
1841-19-6 |
| Literature: Tetrahedron, , vol. 60, # 50 p. 11487 - 11492 |
|
~% 
1841-19-6 |
| Literature: Tetrahedron, , vol. 60, # 50 p. 11487 - 11492 |
|
~% 
1841-19-6 |
| Literature: Tetrahedron, , vol. 57, # 8 p. 1631 - 1637 |
|
~% 
1841-19-6 |
| Literature: Tetrahedron, , vol. 57, # 8 p. 1631 - 1637 |
|
~% 
1841-19-6 |
| Literature: Tetrahedron, , vol. 57, # 8 p. 1631 - 1637 |
|
~% 
1841-19-6 |
| Literature: Tetrahedron, , vol. 57, # 8 p. 1631 - 1637 |
| Precursor 8 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |