600-57-7
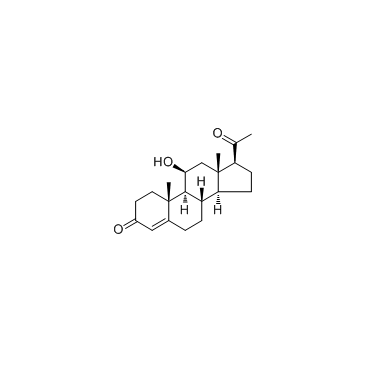
| Name | 11β-hydroxyprogesterone |
|---|---|
| Synonyms |
11BETA-HYDROXYPROGESTERONE
4-PREGNEN-11BETA-OL-3,20-DIONE 21-deoxycorticosterone 11B-HYDROXYPROGESTERONE HYDROXYPROGESTERONE,11B 11beta-hydroxyprogesterone 4-PREGNEN-11BETA-3,20-DIONE |
| Description | 11beta-Hydroxyprogesterone is a potent inhibitors of 11β-Hydroxysteroid dehydrogenase; also activates human mineralocorticoid receptor in COS-7 cells with an ED50 of 10 nM. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | 11OHP displays agonist mineralocorticoid activity. 11β-hydroxyprogesterone activates the transiently expressed hMR in COS-7 cells in a dose-dependent manner with an ED50 of 10 nM and stimulates Ams/sc in mpkCCDcl4 cells. Docking 11β-hydroxyprogesterone within the hMR-ligand-binding domain homology model reveals that the agonist activity of 11OHP is caused by contacts between its 11β-hydroxyl group and Asn770[1]. |
| In Vivo | 11β-hydroxyprogesterone causes a significant elevation in blood pressure within 3 days, an effect that persisted throughout the 14-day infusion. 11β-hydroxyprogesterone is potently hypertensinogenic in the rat and that this activity depends on an intact adrenal and at least in part on the activation of mineralocorticoid receptors[2]. |
| Animal Admin | Rats: 11α- and 11β-OHP are dissolved in propylene glycol (100%) and infused at 3 and 10 μg/h, respectively, for 14 days. Control rats received vehicle only. BP is measured the day before pumps were implanted and on days 3, 7, 10, and 14 after implantation. Indirect systolic BPs are measured with a modified tail-cuff method[2]. |
| References |
| Density | 1.15g/cm3 |
|---|---|
| Boiling Point | 487.4ºC at 760 mmHg |
| Molecular Formula | C21H30O3 |
| Molecular Weight | 330.46100 |
| Flash Point | 262.7ºC |
| Exact Mass | 330.21900 |
| PSA | 54.37000 |
| LogP | 3.69430 |
| Index of Refraction | 1.557 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 9 | |
|---|---|
| DownStream 2 | |