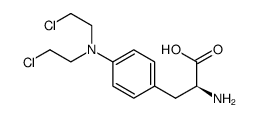
26367-45-3
| Name | DL-Alanine, 3-[p-bis(2-chloroethyl)aminophenyl]-N-formyl |
|---|---|
| Synonyms |
Formyl-L-valylglycin
N-formylsarcolysine N-formyl-sarcolysin N-[N-formyl-L-valyl=>glycine FOR-PHENYLALANINE N-FORMYL-L-PHENYLALNANINE N-formylmelphalan (+)-(S)-N-formylphenylalanine N-formyl-3-phenyl-L-alanine N-Formyl-L-valyl-glycin N-formylphenylalanine (S)-2-formylamino-3-phenylpropionic acid 4-[Bis-(2-chlor-aethyl)-amino]-N-formyl-phenylalanin L-(HCO)-Val-Gly-OH FOR-PHE-OH FORMYL-L-PHENYLALANINE 4-[bis-(2-chloro-ethyl)-amino]-N-formyl-phenylalanine |
| Description | N-Formylsarcolysine has antitumor activity, and inhibits leukemia by increasing the Hb and erythrocyte levels and decreasing the number of leukocytes. N-Formylsarcolysine also involves in glioblastoma and other diseases research[1][2]. |
|---|---|
| Related Catalog | |
| References |
[2]. ChenYY, et al. Quinoline derivative for treating glioblastoma: China, CN112294814[P]. 2021-02-02. |
| Molecular Formula | C14H18Cl2N2O3 |
|---|---|
| Molecular Weight | 333.21000 |
| Exact Mass | 332.06900 |
| PSA | 69.64000 |
| LogP | 2.73910 |
|
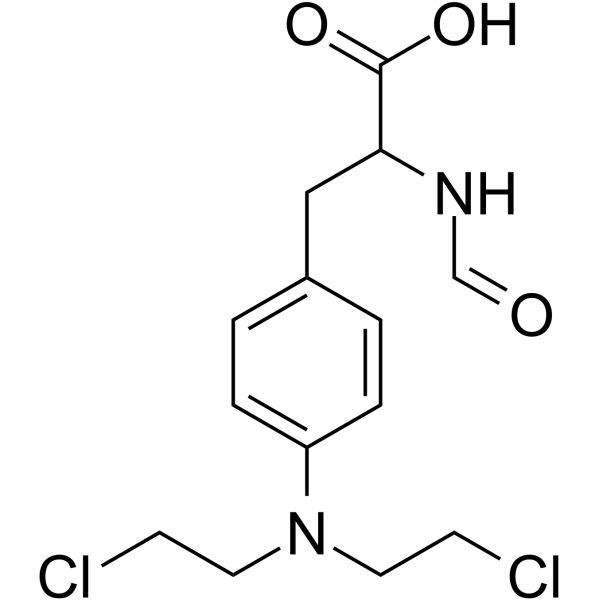
~% 
26367-45-3 |
| Literature: Jin, Guang-Zhu; You, Young-Jae; Ahn, Byung-Zun Bioorganic and Medicinal Chemistry Letters, 2001 , vol. 11, # 11 p. 1473 - 1476 |
|
~% 
26367-45-3 |
| Literature: Schkodinskaja et al. Zhurnal Obshchei Khimii, 1959 , vol. 29, p. 3776; engl. Ausg. S. 3736 Full Text Show Details Knunjanz et al. Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1956 , p. 1418; engl. Ausg. S. 1455 |