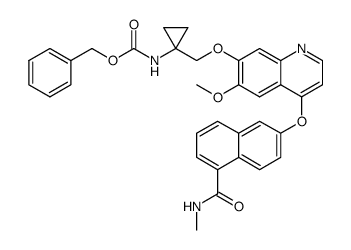
1058137-23-7
| Name | 6-[7-[(1-aminocyclopropyl)methoxy]-6-methoxyquinolin-4-yl]oxy-N-methylnaphthalene-1-carboxamide |
|---|---|
| Synonyms |
CS-0782
Lucitanib QC-4603 UNII-PP449XA4BH E-3810 amine 6-[[7-[(1-aminocyclopropyl)methoxy]-6-methoxy-4-quinolyl]oxy]-N-methyl-naphthalene-1-carboxamide 6-(7-((1-Aminocyclopropyl)methoxy)-6-methoxyquinolin-4-yloxy)-N-methyl-1-naphthamide Lucitanib [INN] E-3810 |
| Description | Lucitanib (E-3810) is a novel dual inhibitor of VEGFR and FGFR, potently and selectively inhibits VEGFR1, VEGFR2, VEGFR3, FGFR1 and FGFR2 with IC50s of 7 nM, 25 nM, 10 nM, 17.5 nM, and 82.5 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
VEGFR1:7 nM (IC50) VEGFR2:25 nM (IC50) VEGFR3:10 nM (IC50) FGFR1:17.5 nM (IC50) FGFR2:82.5 nM (IC50) |
| In Vitro | Consistent with the inhibitory activity of VEGFR and FGFR auto-phosphorylation, Lucitanib potently inhibits VEGF and bFGF-stimulated HUVEC proliferation with IC50 of 40 and 50 nM, respectively. Besides, Lucitanib (E-3810) also inhibits CSF-1R with IC50 of 5 nM[1]. Lucitanib potently inhibits FGFR2 activity (Ki<0.05 μM), follows by PDGFRα activity (Ki=0.11 μM). The Ki values obtained for DDR2, LYN, CARDIAK, CSBP (2), EPHA2, and YES range between 0.26 and 8 μM[2]. |
| In Vivo | Lucitanib (E-3810), at oral dosing of 20 mg/kg for 7 consecutive days, completely inhibits (P<0.01) the bFGF induced angiogenic response compare with the response in vehicle-treated mice. Lucitanib (E-3810) shows a broad spectrum of activity, being active in all the xenografts tested (HT29 colon carcinoma, A2780 ovarian carcinoma, A498, SN12K1, and RXF393 renal carcinomas) with dose-dependent inhibition of tumor growth. E-3810 significantly delays growth during treatment, but tumors resume their growth when treatment is suspended; in a few cases, tumor regression is observed[1]. The activity of Lucitanib (E-3810) given at the doses of 15 mg/kg is tested on MDA-MB-231 breast cancer transplanted subcutaneously, at a late stage, when tumor masses reach 350 to 400 mg. This tumor xenograft is very sensitive to Lucitanib (E-3810), with complete tumor stabilization lasting throughout the 30-day treatment. As in other tumor models, tumors re-grow after withdrawal of Lucitanib (E-3810) at a rate similar to control tumors[3]. |
| Cell Assay | Exponentially growing HUVEC or NHI3T3 cells are seeded into 96-well plates at a density of 3 to 6×103 cells/100 μL/well in complete medium. In the experiments without serum starvation, 24 hours after seeding, cells are exposed to different Lucitanib (E-3810) concentrations without or with VEGF165 (50 ng/mL) or bFGF (20 ng/mL) ligands and the antiproliferative effect of the drugs is evaluated after 72 hours by MTS Colorimetric Assay. In the assays with serum starvation conditions, 24 hours after seeding complete medium is removed and after 3 rounds of washing with PBS, cells are cultured in medium containing 1% BSA. After 18 to 24 hours, cells are processed. Exponentially growing A2780, A498, SN12KI, and HepG2 cells are seeded into 96-well plates at 3 to 5×103 cells/100 μL/well in complete medium. Twenty-four hours later cells are treated with different drug concentrations for 72 hours and the antiproliferative effect is evaluated by MTS[1]. |
| Animal Admin | Mice[3] MDA-MB-231 tumor-bearing mice are randomized when their tumor masses are about 350 to 400 mg to receive Lucitanib (E-3810) (15 mg/kg), Brivanib, and Sunitinib at the doses used for the antitumor activity trial, for 10 days. Four hours after the antiangiogenic dose of day 7, Paclitaxel is injected intravenously at the dose of 20 mg/kg and tumor and plasma samples are collected after 1, 4, and 24 hours in all the groups (each group consisting of 3 animals). At the indicated sampling time, mice are anesthetized, blood is collected from the retro-orbital plexus into heparinized tubes, and the plasma fraction is separated. Mice are killed by cervical dislocation, and tumors excised and snap-frozen. The samples are analyzed by high-performance liquid chromatography (HPLC) with UV detection at 230 nm. |
| References |
| Molecular Formula | C26H25N3O4 |
|---|---|
| Molecular Weight | 443.49400 |
| Exact Mass | 443.18500 |
| PSA | 99.19000 |
| LogP | 5.69370 |
| Storage condition | 2-8℃ |
|
~77% 
1058137-23-7 |
| Literature: EOS ETHICAL ONCOLOGY SCIENCE S.p.A. in abbreviated form EOS S.p.A.; SPINELLI, Silvano; LIVI, Valeria Patent: WO2010/105761 A1, 2010 ; Location in patent: Page/Page column 21 ; |