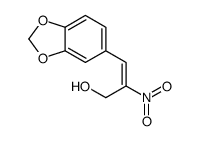
1485-00-3
| Name | 3,4-Methylenedioxy-beta-nitrostyrene |
|---|---|
| Synonyms |
1,3-Benzodioxole, 5- (2-nitroethenyl)-
3,4-Methylenedioxy-β-nitrostyrene 5-[(E)-2-Nitrovinyl]-1,3-benzodioxole 1,3-Benzodioxole, 5-[(E)-2-nitroethenyl]- T56 BO DO CHJ G1U1NW MFCD00014575 5-(2-Nitrovinyl)benzo[d][1,3]dioxole MNS |
| Description | MNS is a potent and selective inhibitor of Src and Syk tyrosine kinases. target: src, syk. [1]IC50:29.3 (src), 2.5 uM (syk); [1]In vitro: no direct effects on protein kinase C, Ca2+ mobilization, Ca2+-dependent enzymes, PKC activation. MNS potently prevents GPIIb/IIIa activation and platelet aggregation without directly affecting other signaling pathways required for platelet activation. [1] [2] MNS is much more potent than genistein in inhibiting platelet aggregation and protein tyrosine phosphorylation. [2] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 334.9±11.0 °C at 760 mmHg |
| Melting Point | 159-163 °C |
| Molecular Formula | C9H7NO4 |
| Molecular Weight | 193.156 |
| Flash Point | 168.8±21.3 °C |
| Exact Mass | 193.037506 |
| PSA | 64.28000 |
| LogP | 2.27 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.640 |
| Storage condition | Store at +4°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn:Harmful; |
|---|---|
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S36/37/39-S26-S45 |
| RIDADR | UN 2811 6.1/PG 3 |
| RTECS | WL5270000 |
| HS Code | 2932999099 |
|
~99% 
1485-00-3 |
| Literature: McNulty, James; Steere, Jennifer A.; Wolf, Sonja Tetrahedron Letters, 1998 , vol. 39, # 44 p. 8013 - 8016 |
|
~87% 
1485-00-3 |
| Literature: Manna, Srimanta; Jana, Sandipan; Saboo, Tapish; Maji, Arun; Maiti, Debabrata Chemical Communications, 2013 , vol. 49, # 46 p. 5286 - 5288 |
|
~80% 
1485-00-3 |
| Literature: Abbott Laboratories Patent: US6162927 A1, 2000 ; US 6162927 A |
|
~71% 
1485-00-3 |
| Literature: Hirao, Haruna; Yamauchi, Satoshi; Ishibashi, Fumio Bioscience, Biotechnology and Biochemistry, 2007 , vol. 71, # 3 p. 741 - 745 |
|
~31% 
1485-00-3 |
| Literature: Research on Chemical Intermediates, , vol. 39, # 8 p. 3715 - 3725 |
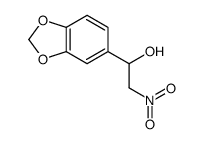
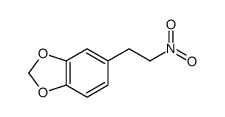
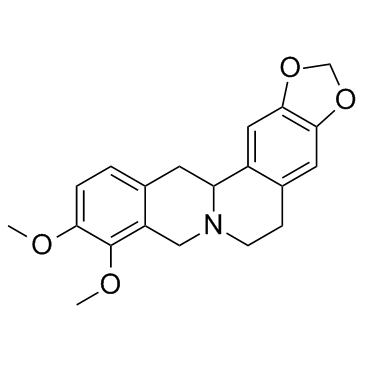
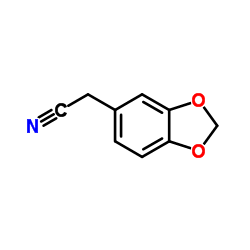
| Precursor 4 | |
|---|---|
| DownStream 9 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |

![7,8-Dihydro-1,3-dioxolo[4,5-g]isoquinoline structure](https://image.chemsrc.com/caspic/152/6882-28-6.png)
![5-[2-(1,3-benzodioxol-5-yl)ethynyl]-1,3-benzodioxole structure](https://image.chemsrc.com/caspic/494/79238-83-8.png)