17318-31-9
| Name | 9-(Tetrahydrofuran-2-yl)-9H-purin-6-amine |
|---|---|
| Synonyms |
9-(Tetrahydro-2-furanyl)-9H-purin-6-amine,9-THF-Ade
9H-Purin-6-amine, 9-(tetrahydro-2-furanyl)- 9-(oxolan-2-yl)purin-6-amine 9-(tetrahydrofuran-2-yl)-9h-purin-6-amine 9-(Tetrahydrofuryl)adenine MFCD00210216 9-(Tetrahydro-2-furanyl)-9H-purin-6-amine SQ 22536 SQ22536 |
| Description | SQ22536 is an effective adenylate cyclase (AC) inhibitor. |
|---|---|
| Related Catalog | |
| Target |
adenylate cyclase (AC)[1] |
| In Vitro | SQ22536 (SQ22,536) effectively inhibits the effect of forskolin with respective IC50 values of 5 μM.Preincubation with graded concentrations of SQ22536 reveals that both SQ22536 effectively inhibits PACAP-induced reporter gene activation with approximate IC50 value of 5 μM. SQ22536 more potently inhibits forskolin-induced Elk activation (IC50=10 μM) than 8-Br-cAMP-induced Elk activation (IC50=170 μM). Most notably, there are substantial differences in the reported potencies of SQ22536 to inhibit the activities of recombinant AC5 and AC6, with respective IC50 values of 2 μM and 360 μM. At a greater concentration (500 μM), SQ22536 significantly inhibits neurite elongation due to either forskolin or 8-Br-cAMP[1]. |
| Cell Assay | HEK293 CRE-luc2P GloResponse luciferase reporter cells are transduced with retroviral vectors expressing rat PAC1hop receptors. Individual cell lines are obtained by limiting dilution cloning, and a clonal PAC1-expressing line is propagated and used for CRE luciferase assays. In brief, HEK293 CRE-luc2P cells are plated in 96-well plates (10,000 cells in 80 μL media per well) in assay media (DMEM supplemented with 1% fetal bovine serum). One day after plating, cells are treated with AC inhibitors (10 μL in assay media/well) for 30 minutes, followed by agonists (10 μL in assay media/well), and are incubated for 4 hours. Luciferase activity is determined after the addition of 100 μL/well Bright-Glo Luciferase Assay Reagent. Luminescence (RLU) is measured in a Victor3 microtiter plate reader after 2 minutes of agitation at room temperature. Cyclic AMP is measured in NS-1 cells. In brief, NS-1 cells are seeded and grown overnight in 96-well plates. The next day, cells are pretreated for 20 minutes in media containing the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (0.5 mM) with or without SQ22536. After pretreatment with inhibitors, cells are stimulated with agonists, added as 10× solutions, for an additional 20 minutes. Intracellular cAMP is then assayed using the cAMP Biotrak enzyme immunoassay kit for measurement of nonacetylated cAMP[1]. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 474.8±55.0 °C at 760 mmHg |
| Melting Point | 160-161ºC |
| Molecular Formula | C9H11N5O |
| Molecular Weight | 205.217 |
| Flash Point | 241.0±31.5 °C |
| Exact Mass | 205.096359 |
| PSA | 78.85000 |
| LogP | -0.17 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.831 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2934999090 |
|
~92% 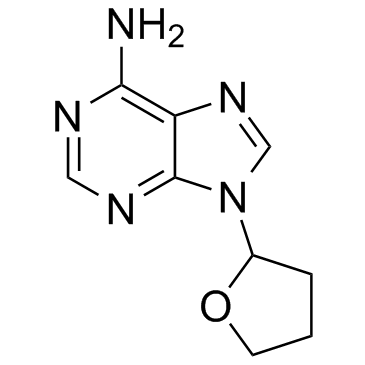
17318-31-9 |
| Literature: Phadtare, Shashikant; Zemlicka, Jiri Journal of the American Chemical Society, 1989 , vol. 111, # 15 p. 5925 - 5931 |
|
~% 
17318-31-9 |
| Literature: Journal of the American Chemical Society, , vol. 111, # 15 p. 5925 - 5931 |
|
~% 
17318-31-9 |
| Literature: Journal of the American Chemical Society, , vol. 111, # 15 p. 5925 - 5931 |
|
~% 
17318-31-9 |
| Literature: Journal of the American Chemical Society, , vol. 111, # 15 p. 5925 - 5931 |
|
~28% 
17318-31-9 |
| Literature: Thompson, Robert D.; Secunda, Sherrie; Daly, John W.; Olsson, Ray A. Journal of Medicinal Chemistry, 1991 , vol. 34, # 9 p. 2877 - 2882 |
|
~% 
17318-31-9 |
| Literature: Heterocycles, , vol. 31, # 2 p. 211 - 214 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |