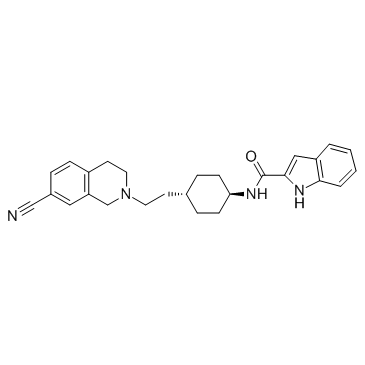
215802-15-6
| Name | SB269652 |
|---|---|
| Synonyms |
1H-Indole-2-carboxamide, N-[trans-4-[2-(7-cyano-3,4-dihydro-2(1H)-isoquinolinyl)ethyl]cyclohexyl]-
N-{trans-4-[2-(7-Cyano-3,4-dihydro-2(1H)-isoquinolinyl)ethyl]cyclohexyl}-1H-indole-2-carboxamide SB269652 |
| Description | SB269652 is the first drug-like allosteric modulator of the dopamine D2 receptor (D2R); a new chemical probe that can differentiate D2R monomers from dimers or oligomers depending on the observed pharmacology.IC50 value: 0.2/0.5 nM [1]Target: D3 receptor antagonistSB269,652 potently (low nanomolar range) abolished specific binding of [(3)H]nemanopride and [(3)H]spiperone to Chinese hamster ovary-transfected D(3) receptors when radioligands were used at 0.2 and 0.5 nM, respectively. However, even at high concentrations (5 μM), SB269,652 only submaximally inhibited the specific binding of these radioligands when they were employed at 10-fold higher concentrations. By analogy, although SB269,652 potently blocked D(3) receptor-mediated activation of Gα(i3) and phosphorylation of extracellular-signal-regulated kinase (ERK)1/2, when concentrations of dopamine were increased by 10-fold, from 1 μM to 10 μM, SB269,652 only submaximally inhibited dopamine-induced stimulation of Gα(i3) [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 690.6±55.0 °C at 760 mmHg |
| Molecular Formula | C27H30N4O |
| Molecular Weight | 426.553 |
| Flash Point | 371.5±31.5 °C |
| Exact Mass | 426.241974 |
| LogP | 5.11 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.662 |
| Storage condition | 2-8℃ |
| RIDADR | NONH for all modes of transport |
|---|