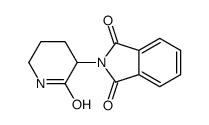
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
TI5635700
-
CHEMICAL NAME :
-
Phthalimide, N-(2-oxo-3-piperidyl)-
-
CAS REGISTRY NUMBER :
-
42472-96-8
-
LAST UPDATED :
-
198910
-
DATA ITEMS CITED :
-
5
-
MOLECULAR FORMULA :
-
C13-H12-N2-O3
-
MOLECULAR WEIGHT :
-
244.27
-
WISWESSER LINE NOTATION :
-
T56 BVNVJ C- CT6MVTJ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1350 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity)
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 31,941,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
747 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity)
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 31,941,1981 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1200 mg/kg
-
SEX/DURATION :
-
female 7-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 31,941,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1200 mg/kg
-
SEX/DURATION :
-
female 7-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 31,941,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1200 mg/kg
-
SEX/DURATION :
-
female 7-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - hepatobiliary system
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 31,941,1981
|