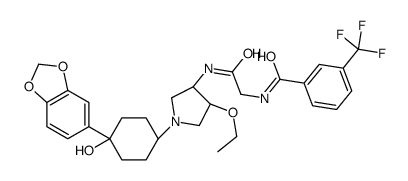
1285539-85-6
| Name | N-[2-({(3R,4R)-1-[4-(1,3-Benzodioxol-5-yl)-4-hydroxycyclohexyl]-4 -ethoxy-3-pyrrolidinyl}amino)-2-oxoethyl]-3-(trifluoromethyl)benz amide |
|---|
| Description | cis-INCB3344 is the inactive isomer of INCB3344 (HY-50674), and can be used as an experimental control. INCB3344 is a potent, selective and orally bioavailable CCR2 antagonist with IC50 values of 5.1 nM (hCCR2) and 9.5 nM (mCCR2) in binding antagonism and 3.8 nM (hCCR2) and 7.8 nM (mCCR2) in antagonism of chemotaxis activity. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C29H34F3N3O6 |
|---|---|
| Molecular Weight | 577.59 |
| Exact Mass | 577.24000 |
| PSA | 116.34000 |
| LogP | 4.55280 |