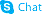279215-43-9
| Name | 2H-Thieno(3,2-E)-1,2,4-thiadiazin-3-amine, 6-chloro-N-(1-methylcyclopropyl)-, 1,1-dioxide |
|---|
| Description | Tifenazoxide (NN414) is a potent, orally active and SUR1/Kir6.2 selective KATP channels opener. Tifenazoxide has antidiabetic effect, can inhibit glucose stimulated insulin release in vitro and in vivo, and has a beneficial effect on glucose homeostasis[1][2]. |
|---|---|
| Related Catalog | |
| Target |
KATP channels[1][2] |
| In Vitro | Tifenazoxide (NN414) hyperpolarises βTC3 cell membranes, and inhibits insulin release from βTC6, from isolated rat islets and from human islets at least a 100-fold more potent than Diazoxide[2]. The EC50 values for Tifenazoxide and diazoxide are 0.45 and 31 µM, respectively in the patch-clamp assay. Tifenazoxide (100 µM) activates Kir6.2/SUR1 channels, but not Kir6.2/SUR2A or Kir6.2/SUR2 channels, expressed in Xenopus oocytes both in whole-cell and inside-out macropatch recordings[2]. Tifenazoxide is a potent inhibitor of glucose stimulated insulin release from βTC6 cells (IC50 = 0.15 µM)[1]. |
| In Vivo | Tifenazoxide (NN414; 1.5 mg/kg; oral administration; twice daily; for 3 weeks; male VDF Zucker (fa/fa) rat) treatment for 3 weeks in VDF rats reduces basal hyperglycemia, improves glucose tolerance, and reduces hyperinsulinemia during an oral glucose tolerance test (OGTT) and improves insulin secretory responsiveness ex vivo[1]. Animal Model: Male Vancouver diabetic fatty (VDF) Zucker rat[1] Dosage: 1.5 mg/kg Administration: Oral administration; twice daily; for 3 weeks Result: Basal glucose was significantly reduced with the fall averaging 0.64 mM after 3 weeks of treatment. |
| References |
| Molecular Formula | C9H10ClN3O2S2 |
|---|---|
| Molecular Weight | 291.77800 |
| Exact Mass | 290.99000 |
| PSA | 107.18000 |
| LogP | 3.05910 |
| Vapour Pressure | 3.22E-08mmHg at 25°C |
| RIDADR | NONH for all modes of transport |
|---|