133804-44-1
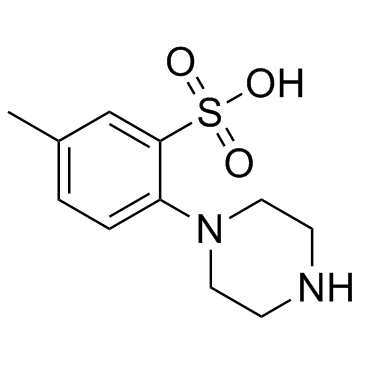
| Name | 5-methyl-2-piperazin-1-ylbenzenesulfonic acid |
|---|---|
| Synonyms |
Caldaret
UNII-9L3Y3IJ2HI MCC 135 |
| Description | Caldaret is an intracellular Ca2+ handling modulator that acts through reverse mode Na+/Ca2+ exchanger inhibition. |
|---|---|
| Related Catalog | |
| Target |
Na+/Ca2+ exchanger[1] |
| In Vitro | Caldaret (MCC-135) is demonstrated to restore Ca2+-ATPase activity of the sarcoplasmic reticulum (SR) isolated from the myocardium acutely exposed to ischemia and reperfusion in vitro[2]. |
| In Vivo | Caldaret, an intracellular Ca2+ handling modulator, limits infarct size of reperfused canine heart. The cardioprotective effect of Caldaret, a novel intracellular Ca2+ handling modulator that acts through reverse-mode Na+/Ca2+ exchanger inhibition and potential sarcoplasmic reticulum (SR) Ca2+ uptake enhancement, against reperfusion injury is investigated. Intravenously infused Caldaret (3 or 30 microg/kg per hour) for 30 min at left circumflex (LCX)-reperfusion markedly reduces infarct size (by 51.3% or 71.9%, respectively). The amelioration of intracellular Ca2+ handling dysfunction achieved by Caldaret leads to cardioprotective effects against reperfusion injury following prolonged ischemia[1]. Caldaret (MCC-135) is a new potent compound with beneficial effects in heart failure. In diabetic rats, Caldaret decreases TR80 significantly without significant effect on developed tension (DT). Caldaret has minimal effects on SR Ca2+ uptake in normal rats, that is observed as increased SR Ca2+ uptake at uptake time of 20 and 30 s at the highest concentration of 10 μM. In diabetic rats, Caldaret increases SR Ca2+ uptake all over the range of uptake time. Both initial rate of SR Ca2+ uptake and the amount of Ca2+ accumulated in the SR with longer uptake time are increased by Caldaret[2]. |
| Animal Admin | Dogs[1] Experiments are performed on 69 male healthy dogs (derived from the hybrid dogs of Labrador retriever, beagle, and hound strain; 6-13 month old; weighing from 10-16 kg). The animals are assigned randomly to four treatment groups: a control group (CONT, n=10) intravenously infused with saline, a low dose of Caldaret-treated group (CAL(L), n=10) infused with Caldaret (3 μg/kg per hour), a high dose of Caldaret-treated group (CAL(H), n=10) infused with Caldaret (30 μg/ kg per hour), and a Diltiazem-treated group (DIL, n=10) infused with Diltiazem (2000 μg/ kg per hour). Saline vehicle or drugs are intravenously administered from 75 min of occlusion to 15 min of reperfusion at a constant rate of 2 mL/kg per hour. Caldaret treatment is designed to evaluate the efficacy against reperfusion injury, thereby the infusion is started 15 min before reperfusion in order to obtain sufficient plasma concentrations at the onset of reperfusion. Caldaret or Diltiazem saline solution is freshly prepared just before the administration in each experimental day. After completion of the successful four hours of reperfusion, ventricular fibrillation (VF) is induced by an intravenous injection of potassium chloride, and the heart is isolated, and processed for postmortem measurement of infarct size and RMBF determinations. |
| References |
| Density | 1.302g/cm3 |
|---|---|
| Molecular Formula | C11H16N2O3S |
| Molecular Weight | 256.32100 |
| Exact Mass | 256.08800 |
| PSA | 78.02000 |
| LogP | 2.12590 |
| Index of Refraction | 1.582 |
| Storage condition | 2-8℃ |