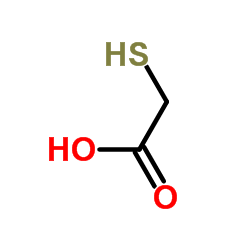
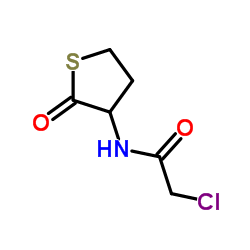
84611-23-4
| Name | Erdosteine |
|---|---|
| Synonyms |
({2-Oxo-2-[(2-oxotetrahydro-3-thiophenyl)amino]ethyl}sulfanyl)acetic acid
N-(Carboxymethylthioacetyl)homocysteine Thiolactone [[2-Oxo-2-[(tetrahydro-2-oxo-3-thienyl)amino]ethyl]thio]acetic Acid ERDOSTEINE BP98 KW-9144 Erdosteine Dithiosteine pv144 ({2-Oxo-2-[(2-oxotetrahydrothiophen-3-yl)amino]ethyl}sulfanyl)acetic acid R,S-erdosteine (±)-[[[(Tetrahydro-2-oxo-3-thienyl)carbamoyl]methyl]thio]acetic Acid Acetic acid, 2-[[2-oxo-2-[(tetrahydro-2-oxo-3-thienyl)amino]ethyl]thio]- RV-144 MFCD00867664 DL-S-[2-[N-3-(2-Oxotetrahydrothienyl)acetamido]]thioglycolic Acid |
| Description | Erdosteine inhibits lipopolysaccharide (LPS)-induced NF-κB activation. |
|---|---|
| Related Catalog | |
| Target |
NF-κB |
| In Vitro | Erdosteine is an oral mucolytic agent used as an expectorant in various chronic respiratory diseases. Erdosteine exerts anti-inflammatory effects by inhibiting NF-κB activation in LPS-stimulated mouse macrophages. However, Erdosteine does not inhibit LPS induced phosphorylation of the Akt and MAPK pathways. To evaluate the toxic effects of Erdosteine on macrophages, cell viability is analyzed. Treatment with 1, 10, or 100 μg/mL Erdosteine does not produce detectable cytotoxicity. Treatment with LPS (1 μg/mL) induced IκBα degradation in RAW 264.7 cells, and maximal degradation is observed after 10 min. RAW 264.7 cells are pretreated with the indicated concentrations of Erdosteine for 6 h and then stimulated with LPS (1 μg/mL) for 10 min. Pretreatment with Erdosteine does not have any effect on the baseline amount of IκBα. Treatment with DMSO alone at a volume equal to that used for Erdosteine delivery does not have any effect on the baseline amount of IκBα. The amount of IκBα is decreased by treatment with LPS for 10 min, and pretreatment with Erdosteine at the indicated concentration and time effectively inhibits IκBα degradation[1]. |
| In Vivo | Twenty-six male mice are divided into four groups as follows: group 1, control; group 2, Erdosteine-treated; group 3, Methotrexate (MTX)-treated; and group 4, Methotrexate+Erdosteine treated. On the first day of experiment, a single dose of Methotrexate is intraperitoneally administered to groups 3 and 4, although a daily single dose of Erdosteine is orally administered to group 2 and 4 for 7 days. At the end of the experiment, the testes of the animals are removed and weighed. The levels of total antioxidant capacity and total oxidative stress, and myeloperoxidase activity in the Methotrexate group are higher than the control group (p<0.05). Lipid peroxidation levels are not changed in Methotrexate group compared with control group. In conclusion, Erdosteine can effectively protect the testes in Methotrexate-induced toxicity. Erdosteine administration with Methotrexate improves testicular injures, as indicated by appearance of spermatogenesis in seminiferous tubules[2]. |
| Cell Assay | The murine macrophage/monocyte cell line RAW264.7 are maintained as monolayers in Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % fetal bovine serum, 60 U/mL Penicillin, and 100 μg/mL Streptomycin at 37.8°C in 5 % CO2. The cell viability is quantified using a colorimetric tetrazolium compound MTS assay. Briefly, 1×104 cells incubated with various concentrations of Erdosteine (1, 10, or 100 μg/mL) for 24 h are treated with 10 μL of MTS solution (5 mg/mL) for 45 min. The cells are then lysed with isopropyl alcohol, and the absorbance is read at the wavelength of 540 nm[1]. |
| Animal Admin | Mice[2] Twenty-six male C57BL/6 mice (8 weeks, 20-30 g) are randomly divided into four groups. In control group (n=6); mice are treated the 0.5 mL of saline as a placebo intraperitoneally (i.p.). In Erdosteine group (n=6), mice are treated with Erdosteine orally (gavage; 10 mg/kg) for 7 days. In this study, low-dose MTX are used because high-dose (20-40 mg/kg) MTX has anti-inflammatory and immunosuppressive activity. Mice in MTX group (n=7) are injected with single dose of i.p. MTX (10 mg/kg). In MTX+Erdosteine group (n=7), mice are injected with single dose of i.p. MTX (10 mg/kg) the first day and Erdosteine is given orally (10 mg/kg) to the animals starting the first day for 7 days. After the last administration of the drug, all rats fasted about 12 hours, but have free access to water. And then, the animals are sacrificed by cervical dislocation at the end of the experiment. Following sacrifice, the testes are quickly removed from the mice. Right testes specimens are fixed in 10% neutral-buffered formaldehyde solution for histological assessment[2]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 590.4±50.0 °C at 760 mmHg |
| Melting Point | 156-160°C |
| Molecular Formula | C8H11NO4S2 |
| Molecular Weight | 249.307 |
| Flash Point | 310.8±30.1 °C |
| Exact Mass | 249.012955 |
| PSA | 134.07000 |
| LogP | -0.32 |
| Vapour Pressure | 0.0±3.6 mmHg at 25°C |
| Index of Refraction | 1.615 |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26-S36/37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2934999090 |
|
~77% 
84611-23-4 |
| Literature: Gobetti; Pedrazzoli; Bradamante Farmaco, Edizione Scientifica, 1986 , vol. 41, # 1 p. 69 - 79 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |