471-95-4
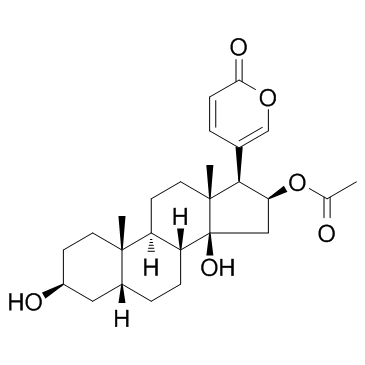
| Name | Bufotaline |
|---|---|
| Synonyms |
Bufotaline
Bufotalin std. 16-(Acetyloxy)-3,14-dihydroxybufa-20,22-dienolide BUFOTALIN(SH) BUFOTALIN SULFATE Bufa-20,22-dienolide, 16-(acetyloxy)-3,14-dihydroxy-, (3β,5β,16β)- (3b,5b,16b)-16-(Acetyloxy)-3,14-dihydroxybufa-20,22-dienolide (3S,5R,8R,9S,10S,13R,14S,16S,17R)-3,14-dihydroxy-10,13-dimethyl-17-(2-oxo-2H-pyran-5-yl)hexadecahydro-1H-cyclopenta[a]phenanthren-16-yl acetate 5β-Bufa-20,22-dienolide, 3β,14,16β-trihydroxy-, 16-acetate (8CI) Bufotalin 3b,14,16b-Trihydroxy-5b-bufa-20,22-dienolide 16-Acetate (3β,5β,16β)-16-Acetoxy-3,14-dihydroxybufa-20,22-dienolide |
| Description | Bufotalin is a cardiotoxic bufanolide steroid, cardiac glycoside analogue, secreted by a number of toad species; a novel anti-osteoblastoma agent.IC50 value:Target:in vitro: bufotalin induced osteoblastoma cell death and apoptosis in dose- and time-dependent manners. Further, bufotalin induced endoplasmic reticulum (ER) stress activation in osteoblastoma cells, the latter was detected by the induction of C/EBP homologous protein (CHOP), phosphorylation of inositol-requiring enzyme 1 (IRE1) and PKR-like endoplasmic reticulum kinase (PERK), as well as caspase-12 activation [1]. Bufotalin was the most potent active compound among these four bufadienolides, and it exerted stronger inhibitory effect on the viability of doxorubicin-induced multidrug resistant liver cancer cells (R-HepG2) than that of their parent cells HepG2. bufotalin treatment induced cell cycle arrest at G(2)/M phase through down-regulation of Aurora A, CDC25, CDK1, cyclin A and cyclin B1, as well as up-regulation of p53 and p21. Bufotalin treatment also induced apoptosis which was accompanied by decrease in mitochondrial membrane potential, increases in intracellular calcium level and reactive oxygen species production, activations of caspase-9 and -3, cleavage of poly ADP-ribose polymerase (PARP) as well as changes in the expressions of bcl-2 and bax [2]. Bufotalin promoted death receptor-mediated cell death, especially TRAIL-induced apoptosis, through activation of caspase-3 and PARP-1. Cotreatment of bufotalin with TRAIL resulted in the downregulation of anti-apoptotic proteins, including Bcl-XL, Mcl-1, survivin and XIAP, and the up-regulation of MAPKs and TRAIL receptor DR5. In addition, phosphorylation of STAT1 was strongly inhibited by bufotalin [3]. externalization of phosphatidylserine, accumulation of sub-G(1) cells, fragmentation of DNA, and formation of apoptotic bodies were observed in bufotalin-treated Hep 3B cells [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 591.7±50.0 °C at 760 mmHg |
| Melting Point | 223°C (rough estimate) |
| Molecular Formula | C26H36O6 |
| Molecular Weight | 444.560 |
| Flash Point | 195.8±23.6 °C |
| Exact Mass | 444.251190 |
| PSA | 96.97000 |
| LogP | 2.54 |
| Vapour Pressure | 0.0±3.8 mmHg at 25°C |
| Index of Refraction | 1.587 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | UN 3172 |
|---|---|
| Packaging Group | II |
| Hazard Class | 6.1(a) |
| HS Code | 29329990 |