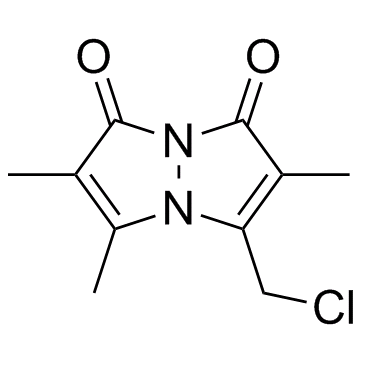
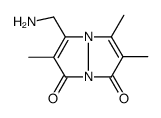
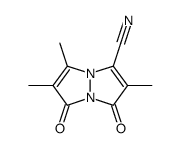
71418-44-5
| Name | monobromobimane |
|---|---|
| Synonyms |
MONOBROMOBIMANE
Monobromobimane Bromobimane |
| Description | Bromobimane is essentially nonfluorescent and converts into fluorescent products when reacts with small thiols. |
|---|---|
| Related Catalog | |
| In Vitro | Bromobimanes in solution (aqueous or organic solvents of medium polarity) react with small thiols [e.g., the tripeptide thiol glutathione (GSH)], and with reactive protein thiol groups (e.g., hemoglobin). The reactions of bromobimanes with thiols are second order and dependent on pH, the active nucleophile being the thiolate anion. The reaction of bromobimane with a thiolate converts the nonfluorescent agent into water-soluble fluorescent products. The neutral agents mBBr and bBBr are moderately soluble in mediumpolarity organic solvents (acetonitrile, dichloromethane), and slightly soluble in water. The quaternary salt, qBBr, and the anionic bromobimane, SBBr, are soluble in water, but less soluble in organic solvents. Bromobimanes are yellow[1]. The highly selective, rapid reactivity of bromobimanes toward thiols, the stability and fluorescence yield of the thiol derivatives, the ease of separation of the derivatives by reversed-phase HPLC, and the availability of both cell-penetrating and nonpenetrating forms, make the use bromobimanes an extremely powerful approach to the analysis of low molecular weight biothiols[2]. |
| Cell Assay | The stock mBBr solution is prepared by weight at 100-180 mM in acetonitrile. The stock solution of SBBr is prepared by weight at 50 mM in dimethyl sulfoxide (DMSO) or at 2 mM in the 20 mM Tris-methane sulfonate. The thiol is added to a final concentration of 1 mM to a solution of 2 mM mBBr or SBBr, 20 mM Tris-methane sulfonate, pH 8.0. The mixture is incubated in the dark for 15 min at room temperature and then methanesulfonic acid is added to 25 mM from a 5 M stock solution[2]. |
| References |
[1]. Kosower EM, et al. Bromobimane probes for thiols. Methods Enzymol. 1995;251:133-48. |
| Density | 1.66g/cm3 |
|---|---|
| Boiling Point | 327.8ºC at 760 mmHg |
| Melting Point | 152-154ºC |
| Molecular Formula | C10H11BrN2O2 |
| Molecular Weight | 271.11100 |
| Flash Point | 152.1ºC |
| Exact Mass | 270.00000 |
| PSA | 42.96000 |
| LogP | 1.01710 |
| Index of Refraction | 1.64 |
| Storage condition | -20℃ |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
|
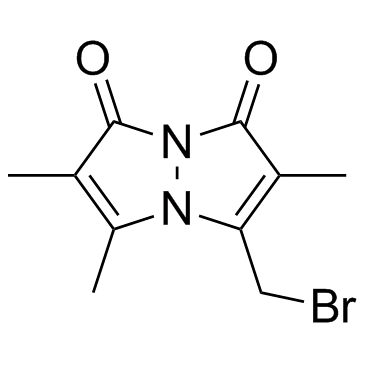
~% 
71418-44-5 |
| Literature: Journal of the American Chemical Society, , vol. 102, p. 4983 |
|
~% 
71418-44-5 |
| Literature: Journal of the American Chemical Society, , vol. 102, p. 4983 |
|
~% 
71418-44-5 |
| Literature: Journal of the American Chemical Society, , vol. 102, p. 4983 |
|
~49% 
71418-44-5 |
| Literature: Journal of the American Chemical Society, , vol. 102, p. 4983 |
|
~38% 
71418-44-5 |
| Literature: Kosower, Edward M.; Pazhenchevsky, Barak; Dodiuk, Hanna; Kanety, Hannah; Faust, Dov Journal of Organic Chemistry, 1981 , vol. 46, # 8 p. 1666 - 1673 |
|
~% 
71418-44-5 |
| Literature: Journal of Organic Chemistry, , vol. 46, # 8 p. 1666 - 1673 |
| Precursor 5 | |
|---|---|
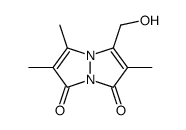
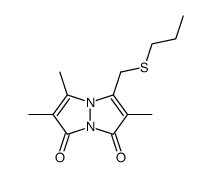
| DownStream 6 | |
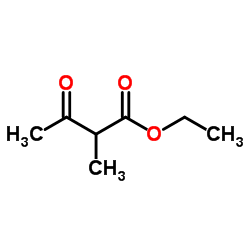

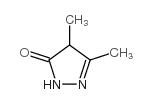
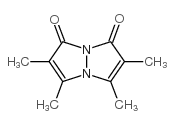
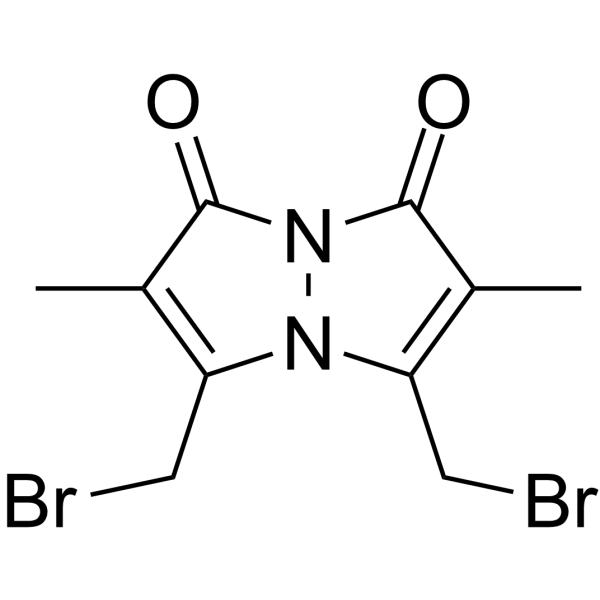
![2-[(1,2,6-trimethyl-3,5-dioxopyrazolo[1,2-a]pyrazol-7-yl)methylsulfanyl]acetic acid structure](https://image.chemsrc.com/caspic/161/119843-35-5.png)