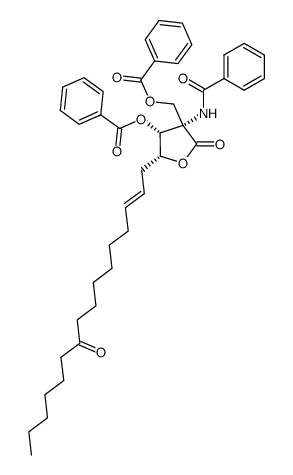
35891-70-4
| Name | myriocin |
|---|---|
| Synonyms |
Thermozymocidin
ISP-1 6-Eicosenoic acid, 2-amino-3,4-dihydroxy-2-(hydroxymethyl)-14-oxo-, (2S,3R,4R,6E)- (2S,3R,4R,6E)-2-Amino-3,4-dihydroxy-2-(hydroxymethyl)-14-oxo-6-icosenoic acid D-Serine, 2-[(1R,2R,4E)-1,2-dihydroxy-12-oxo-4-octadecen-1-yl]- ISP-I Myriocin from Mycelia sterilia MFCD01632772 Myriocin Myriocin,Mycelia sterilia antibiotic ISP-I (E,2S,3R,4R)-2-amino-3,4-dihydroxy-2-(hydroxymethyl)-14-oxoicos-6-enoic acid 2-[(1R,2R,4E)-1,2-Dihydroxy-12-oxo-4-octadecen-1-yl]-D-serine |
| Description | Myriocin, a fungal metabolite isolated from Myriococcum albomyces, Isaria sinclairi and Mycelia sterilia, is a potent inhibitor of serine-palmitoyl-transferase (SPT) and a key enzyme in de novo synthesis of sphingolipids[1]. Myriocin strongly suppresses replication of both the subgenomic HCV-1b replicon and the JFH-1 strain of genotype 2a infectious HCV[2], with an IC50 of 3.5 µg/mL for inhibiting HCV infection[3]. |
|---|---|
| Related Catalog | |
| Target |
Serine-palmitoyl-transferase (SPT)[1] |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 636.7±55.0 °C at 760 mmHg |
| Molecular Formula | C21H39NO6 |
| Molecular Weight | 401.538 |
| Flash Point | 338.8±31.5 °C |
| Exact Mass | 401.277740 |
| PSA | 141.08000 |
| LogP | 4.21 |
| Vapour Pressure | 0.0±4.3 mmHg at 25°C |
| Index of Refraction | 1.522 |
| Storage condition | -20℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xi; Xn |
| Risk Phrases | 22 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3.0 |
| RTECS | JX3890000 |
| Hazard Class | 6.1 |
| Precursor 8 | |
|---|---|
| DownStream 1 | |

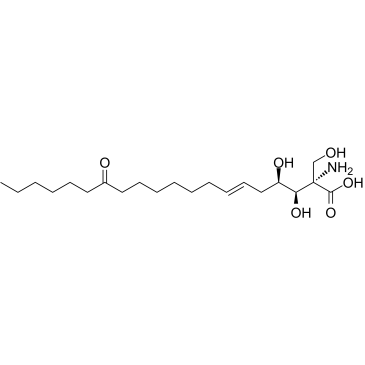
![(2S,3R,4R)-2-acetamido-3-acetoxy-2-acetoxymethyl-4-[(E)-10'-oxohexadec-2'-en-1'-yl]-4-butanolide structure](https://image.chemsrc.com/caspic/444/37818-01-2.png)