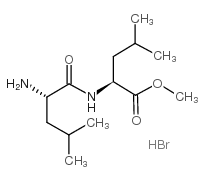
16689-14-8
| Name | Leu-Leu methyl ester hydrobromide |
|---|---|
| Synonyms |
methyl 2-[(2-amino-4-methylpentanoyl)amino]-4-methylpentanoate,hydrobromide
LLME,HBr Leu-Leu-Ome,HBr |
| Description | L-Leucyl-L-Leucine methyl ester (LLOMe) hydrobromide, a dipeptide condensation product of L-leucine methyl ester generated by human monocytes or polymorphonuclear leukocytes, selectively eliminates lymphocytes with cytotoxic potential. L-Leucyl-L-Leucine methyl ester hydrobromide also can induce endolysosomal pathway stress[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | L-Leucyl-L-Leucine methyl ester (1 mM; 0.5-2 h) enhances LRRK2-mediated Rab10 and Rab12 phosphorylation in MEFs and A549 cells[3]. L-Leucyl-L-Leucine methyl ester (10-250 μM; 15 min) is converted to a CCI3COOH-insoluble product by CD4- lymphocytes[2]. |
| References |
| Molecular Formula | C13H27BrN2O3 |
|---|---|
| Molecular Weight | 339.26900 |
| Exact Mass | 338.12100 |
| PSA | 81.42000 |
| LogP | 3.11300 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2924199090 |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |