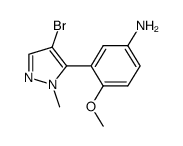
839713-36-9
| Name | 1-[3-(4-bromo-2-methylpyrazol-3-yl)-4-methoxyphenyl]-3-(2,4-difluorophenyl)urea |
|---|---|
| Synonyms |
UNII-4ZA73QEW2P
Nelotanserin APD125 |
| Description | Nelotanserin is a potent 5-HT2A inverse agonist, a moderately potent 5-HT2C partial inverse agonist and a weak 5-HT2B inverse agonist, with IC50s of 1.7, 79, 791 nM in IP accumulation assays, respectively. |
|---|---|
| Related Catalog | |
| Target |
5-HT2A Receptor:1.7 nM (IC50) 5-HT2C Receptor:79 nM (IC50) 5-HT2B Receptor:791 nM (IC50) |
| In Vitro | Results from IP accumulation assays suggest that Nelotanserin is a potent 5-HT2A full inverse agonist (IC50=1.7 nM), a moderately potent 5-HT2C partial inverse agonist (IC50=79 nM) (maximal response was 62% of the response obtained for the reference inverse agonist clozapine), and a weak 5-HT2B inverse agonist (IC50=791 nM). Nelotanserin displays high affinity for recombinant human 5-HT2A receptors (Ki=0.35 nM), moderate affinity for human 5-HT2C receptors (Ki=100 nM), and low affinity for human 5-HT2B receptors (2000 nM) stably expressed in HEK293 cells. The results suggest that Nelotanserin has a 262-fold higher affinity for human 5-HT2A than 5-HT2C receptors and a 6610-fold higher affinity for human 5-HT2A than 5-HT2B receptors[1]. |
| In Vivo | Each compound is tested in a minimum of five rats by oral gavage with administration occurring in the middle of the inactive period, 6 h after light onset. The delta power during non-REM sleep (NREMS) is significantly different between all the analogues tested and the vehicle control. Nelotanserin (Compound 39) produces significant increases in delta power that persist for the first 4 h following dosing. Significant differences are found, however, in NREMS bout length. Nelotanserin significantly increases NREMS bout length during the first hour following dosing, and 3 does so during the second hour. In conjunction with this increased NREM bout duration, the number of NREM bouts decrease during the first hour for Nelotanserin (p<0.01) as well as for compound 15 (p<0.05)[2]. |
| References |
| Density | 1.55 g/cm3 |
|---|---|
| Boiling Point | 425.886ºC at 760 mmHg |
| Molecular Formula | C18H15BrF2N4O2 |
| Molecular Weight | 437.23800 |
| Flash Point | 211.369ºC |
| Exact Mass | 436.03500 |
| PSA | 71.67000 |
| LogP | 4.86700 |
| Storage condition | 2-8℃ |
|
~94% 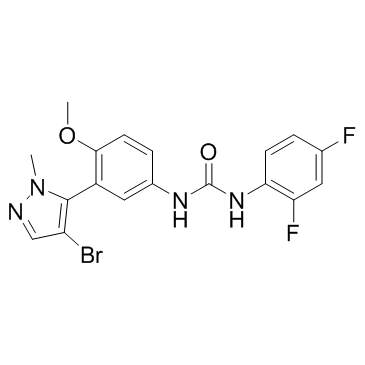
839713-36-9 |
| Literature: ARENA PHARMACEUTICALS, INC.; CARLOS, Marlon V.; DONG, Weitong; MACIAS, Mark; SATO, Suzanne Michiko; SILVEY, Gary Patent: WO2010/62321 A1, 2010 ; Location in patent: Page/Page column 75-76 ; |
|
~% 
839713-36-9 |
| Literature: ARENA PHARMACEUTICALS, INC. Patent: WO2006/81335 A2, 2006 ; Location in patent: Page/Page column 36-38 ; WO 2006/081335 A2 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |