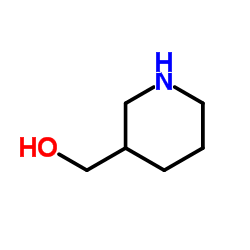
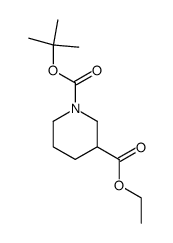
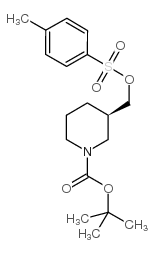
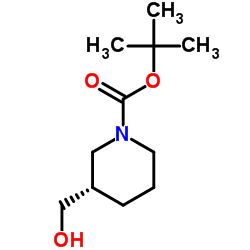
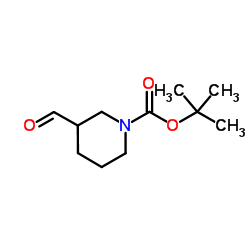
116574-71-1
| Name | N-Boc-piperidine-3-methanol |
|---|---|
| Synonyms |
MFCD02683203
1-Piperidinecarboxylic acid, 3-(hydroxymethyl)-, 1,1-dimethylethyl ester 1-(tert-Butoxycarbonyl)-3-piperidinemethanol 1-(tert-Butoxycarbonyl)-3-(hydroxymethyl)piperidine 1-Boc-3-(hydroxymethyl)piperidine tert-butyl 3-(hydroxymethyl)tetrahydro-1(2H)-pyridinecarboxylate 2-Methyl-2-propanyl 3-(hydroxymethyl)-1-piperidinecarboxylate tert-butyl 3-(Hydroxymethyl)piperidine-1-carboxylate 1-Boc-3-(Hydroxymethyl) Piperidine N-Boc-3-(hydroxymethyl)piperidine tert-Butyl-3-(hydroxymethyl)piperidin-1-carboxylat 1-Boc-3-piperidinemethanol |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 308.0±15.0 °C at 760 mmHg |
| Melting Point | 77-81ºC |
| Molecular Formula | C11H21NO3 |
| Molecular Weight | 215.289 |
| Flash Point | 140.1±20.4 °C |
| Exact Mass | 215.152145 |
| PSA | 49.77000 |
| LogP | 0.84 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.479 |
| Hazard Codes | Xi:Irritant; |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2933399090 |
|
~99% 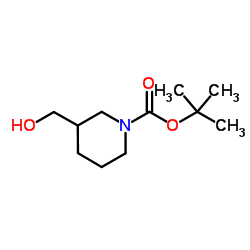
116574-71-1 |
| Literature: Batt, Douglas G. Patent: US2004/67935 A1, 2004 ; Location in patent: Page/Page column 28 ; US 20040067935 A1 |
|
~10% 
116574-71-1 |
| Literature: H. LUNDBECK A/S Patent: US2011/92475 A1, 2011 ; Location in patent: Page/Page column 18 ; |
|
~95% 
116574-71-1 |
| Literature: Bristol-Myers Squibb Company Patent: US5583146 A1, 1996 ; US 5583146 A |
|
~% 
116574-71-1 |
| Literature: US2002/16337 A1, ; |
|
~% 
116574-71-1 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 12, # 21 p. 3235 - 3238 |
|
~% 
116574-71-1 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 12, # 21 p. 3235 - 3238 |
|
~% 
116574-71-1 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 12, # 21 p. 3235 - 3238 |
| Precursor 6 | |
|---|---|
| DownStream 9 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |