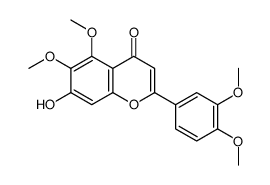
22368-21-4
| Name | eupatilin |
|---|---|
| Synonyms |
5,7-Dihydroxy-3',4',6-trimethoxyflavone
Eupatilin 5,7-dihydroxy-6,3',4'-trimethoxyflavone 2-(3,4-Dimethoxyphenyl)-5,7-dihydroxy-6-methoxy-4H-chromen-4-one 4H-1-Benzopyran-4-one, 2-(3,4-dimethoxyphenyl)-5,7-dihydroxy-6-methoxy- Euptailin 2-(3,4-dimethoxyphenyl)-5,7-dihydroxy-6-methoxychromen-4-one |
| Description | Eupatilin, a lipophilic flavonoid isolated from Artemisia species, is a PPARα agonist, and possesses anti-apoptotic, anti-oxidative and anti-inflammatory activities. |
|---|---|
| Related Catalog | |
| Target |
PPARα |
| In Vitro | Eupatilin is a PPARα agonist. Eupatilin (10, 30, 100 μM) suppresses IL-4 expression and degranulation in RBL-2H3 cells[1]. Eupatilin (50-100 μM) slightly reduces cell viability of HaCaT cells. Eupatilin (10, 30, 50, 100 μM) increases PPARα transactivation and expression in HaCaT cells. Eupatilin (10, 30, 50 μM) also suppresses TNFα-induced MMP-2/-9 expression in HaCaT cells. Furthermore, Eupatilin inhibits TNFα-induced p65 translocation, IκBα Phosphorylation, AP-1 and MAPK signaling via PPARα[2]. Eupatilin (10-50 μM) shows no cytotoxic effects on ARPE19 cells. Eupatilin (10, 25, 50 μM) elevates cell viability from oxidative stress, and inhibits H2O2-induced ROS production in ARPE19 cells. Moreover, Eupatilin (50 μM) inbibits H2O2-induced cells apoptosis and promotes the activation of PI3K/Akt pathway in RPE cells[3]. |
| In Vivo | Eupatilin (1.5% or 3.0%) restores PPARα mRNA expression, and improves atopic dermatitis (AD)-like symptoms in oxazolone-induced Balb/c mice. Eupatilin causes significant decrease in serum IgE, IL-4 levels, oxazolone-induced TNFα, IFNγ, IL-1β, TSLP, IL-33 and IL-25 mRNA expression in oxazolone-induced mice. Eupatilin also increases filaggrin and loricrin mRNA expression in oxazolone-induced mice[1]. |
| Cell Assay | Cell viability is detected using a MTT assay. In brief, after treatment, the medium is replaced with fresh medium containing 0.5 mg/mL MTT for 4 h at 37°C. Then, the medium is gently aspirated and 150 μL of DMSO is added to each well to solubilize the formazan crystals. The absorbance is measured at 450 nm by a microplate reader. The relative cell viability is defined as the absorbance of treated wells divided by that of the control[3]. |
| Animal Admin | Six-week-old female Balb/c mice are housed under conditions of controlled temperature (23 ± 2 °C), humidity (55 ± 5%), and 12 h light/dark cycles (06:00-18:00 h light, 18:00-06:00 dark). Briefly, Balb/c mice are sensitized on day −7 by a single application of 20 μL of 1.0% oxazolone in a mixture of acetone and olive oil (4:1) to the inner and outer surface of both ears. On day 0, the mouse ears are challenged with 20 μL of 0.1% oxazolone at 2-day intervals for 4 weeks post-sensitization. The mice are treated with the indicated concentrations of Eupatilin (1.5% or 3.0%) twice a day for 4 weeks. The control group is treated with vehicle alone (acetone and olive oil [4:1]). After 3 weeks, the mice are sacrificed and samples are collected. Ears are stored at −80 °C for RNA isolation and analysis or immediately fixed in 4% formalin for histological analysis[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 583.6±50.0 °C at 760 mmHg |
| Melting Point | 236 °C |
| Molecular Formula | C18H16O7 |
| Molecular Weight | 344.315 |
| Flash Point | 214.7±23.6 °C |
| Exact Mass | 344.089600 |
| PSA | 98.36000 |
| LogP | 2.46 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.627 |
| HS Code | 2932999099 |
|---|
|
~72% 
22368-21-4 |
| Literature: Dong a Pharmaceutical Co., Ltd. Patent: US6025387 A1, 2000 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |