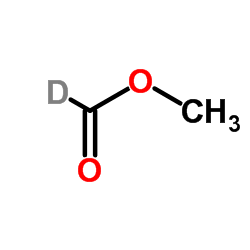
35692-88-7
| Name | deuterioformamide |
|---|---|
| Synonyms |
(H)Formamide
C-Deuterio-formamid d1-N,N-formamide Formamide-1-d Formamide-d formamide-d1 MFCD01074135 C-deuterio-formamide |
| Description | Formamide-d1 is the deuterium labeled Formamide[1]. Formamide is an amide derived from formic acid and has been used as solvent for many ionic compounds. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 210.5±9.0 °C at 760 mmHg |
| Melting Point | 2-3ºC(lit.) |
| Molecular Formula | CH2DNO |
| Molecular Weight | 46.047 |
| Flash Point | 81.1±18.7 °C |
| Exact Mass | 46.027740 |
| PSA | 43.09000 |
| LogP | -1.51 |
| Vapour Pressure | 0.2±0.4 mmHg at 25°C |
| Index of Refraction | 1.370 |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H351-H360D-H373 |
| Precautionary Statements | P201-P260-P280-P308 + P313 |
| Target Organs | Blood |
| Hazard Codes | T: Toxic; |
| Risk Phrases | 61-37/38-41 |
| Safety Phrases | 53-23-26-36/37/39-45 |
| RIDADR | NONH for all modes of transport |
|
~97% 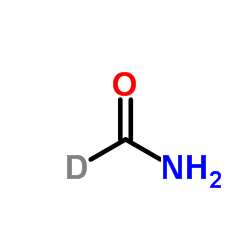
35692-88-7 |
| Literature: Threadgill, Michael D.; Gledhill, Adrian P. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1986 , p. 873 - 876 |
|
~% 
35692-88-7 |
| Literature: Ha, Jeong-Hyon; Kim, Yung Sam; Hochstrasser, Robin M. Journal of Chemical Physics, 2006 , vol. 124, # 6 art. no. 064508 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |