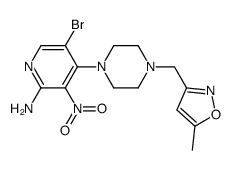
1095382-05-0
| Name | 3-[[4-[6-bromo-2-[4-(4-methylpiperazin-1-yl)phenyl]-1H-imidazo[4,5-b]pyridin-7-yl]piperazin-1-yl]methyl]-5-methyl-1,2-oxazole |
|---|---|
| Synonyms |
3-((4-(6-bromo-2-(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)piperazin-1-yl)methyl)-5-methylisoxazole
6-Bromo-7-{4-[(5-methyl-1,2-oxazol-3-yl)methyl]-1-piperazinyl}-2-[4-(4-methyl-1-piperazinyl)phenyl]-1H-imidazo[4,5-b]pyridine cc-66 1H-Imidazo[4,5-b]pyridine, 6-bromo-7-[4-[(5-methyl-3-isoxazolyl)methyl]-1-piperazinyl]-2-[4-(4-methyl-1-piperazinyl)phenyl]- CS-0706 CCT137690 CCT 137690 |
| Description | CCT 137690 is a potent and orally available aurora kinase inhibitor with IC50s of 15, 25, and 19 nM for aurora A, B and C, respectively. |
|---|---|
| Related Catalog | |
| Target |
Aurora A:15 nM (IC50) Aurora B:25 nM (IC50) Aurora C:19 nM (IC50) |
| In Vitro | CCT 137690 displays antiproliferative activity in a range of human tumor cell lines, including SW620 colon carcinoma (GI50=0.30 μM) and A2780 ovarian cancer cell line (GI50=0.14 μM). CCT 137690 inhibits in vitro phosphorylation of histone H3. CCT 137690 is a moderate inhibitor of the hERG ion-channel (IC50=3.0 μM)[1]. CCT137690 efficiently inhibits histone H3 and TACC3 phosphorylation (Aurora B and Aurora A substrates, respectively) in HCT116 and HeLa cells. Continuous exposure of tumour cells to the inhibitor causes multipolar spindle formation, chromosome misalignment, polyploidy and apoptosis[2]. |
| In Vivo | CCT 137690 slows the growth of the SW620 xenografts with no observed toxicity[1]. CCT 137690 significantly inhibits tumour growth in a transgenic mouse model of neuroblastoma (TH-MYCN) that overexpresses MYCN protein and is predisposed to spontaneous neuroblastoma formation[2]. |
| Cell Assay | Cells are plated in 96-well plates at 3,000 cells per well and are treated with a range of 0 to 25 mol/L of CCT137690 for 72 h. Cell proliferation assays are performed by colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)[2]. |
| Animal Admin | Mice: Animals are randomized into two groups, group 1: treatment with 100 mg/kg CCT137690 n=4 or group 2: vehicle control n=4. Treatment is administered via oral gavage twice daily. Tumour volumes are measured at day 0, 3 (48 hours after treatment started), 7 and 10 using 1H MRI[2]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 722.9±70.0 °C at 760 mmHg |
| Molecular Formula | C26H31BrN8O |
| Molecular Weight | 551.481 |
| Flash Point | 391.0±35.7 °C |
| Exact Mass | 550.180420 |
| PSA | 80.56000 |
| LogP | 3.07 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.664 |
| Storage condition | -20℃ |
| RIDADR | NONH for all modes of transport |
|---|
|
~31% 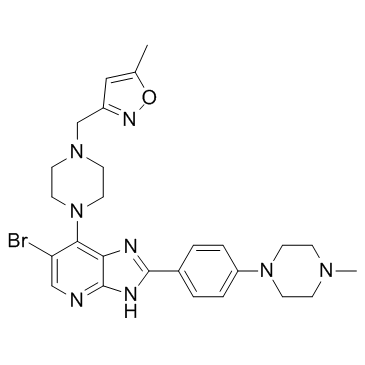
1095382-05-0 |
| Literature: WO2009/1021 A1, ; Page/Page column 61-62 ; WO 2009/001021 A1 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |